SOF/VEL in liver transplantation with genotype 1-4 infection
Agarwal K, J Hepatol 2018; May 29 (ePub ahead of print)
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1
3
1
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Special population
Liver transplantation
Liver transplantation
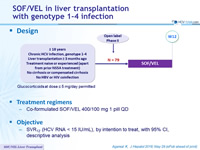
Design
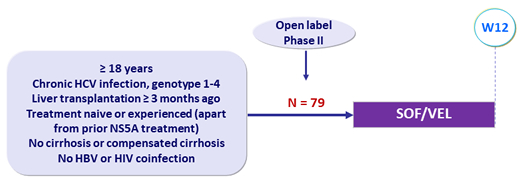
Glucocorticoids at dose = 5 mg/day permitted
Treatment regimens
- Co-formulated SOF/VEL 400/100 mg 1 pill QD
Objective
- SVR12 (HCV RNA < 15 IU/mL), by intention to treat, with 95% CI, descriptive analysis
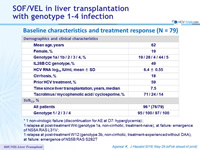
Baseline characteristics and treatment response (N = 79)
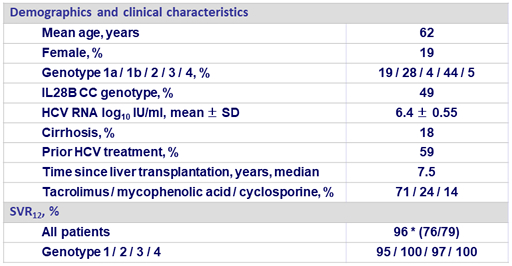
* 1 non-virologic failure (discontinuation for AE at D7: hyperglycemia) ; 1 relapse at post-treatment W4 (genotype 1a, non-cirrhotic, treatment-naive), at failure: emergence of NS5A RAS L31V ; 1 relapse at post-treatment W12 (genotype 3b, non-cirrhotic, treatment-experienced without DAA), at failure: emergence of NS5B RAS S282T
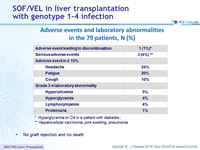
Adverse events and laboratory abnormalities in the 79 patients, N (%)
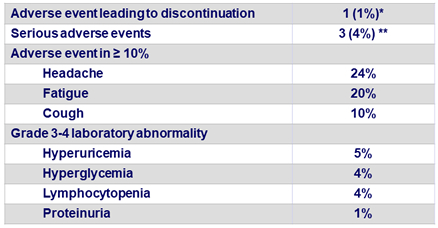
* Hyperglycemia on D4 in a patient with diabetes ; ** Hepatocellular carcinoma, joint swelling, pneumonia
- No graft rejection and no death
Summary
- The single-tablet regimen of SOF/VEL was well tolerated and highly effective in genotype 1-4 HCV-infected liver transplant recipients.
- SOF/VEL is a pangenotypic , ribavirin-free, simple, and well-tolerated treatment option for HCV-infected liver transplant recipients.