RESOLVE study: SOF/VEL/VOX 12 weeks in treatment experienced patients
Covert E. AASLD 2018, Abs. 0583
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1
1a
1b
1
1a
1b
Treatment history
IFN-Experienced
NS5A experienced
SOF-experienced
IFN-Experienced
NS5A experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
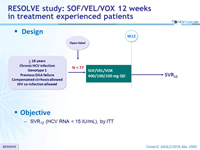
Design
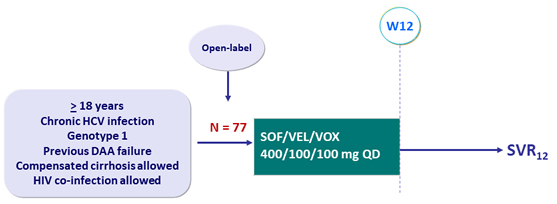
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT
Baseline characteristics and SVR12
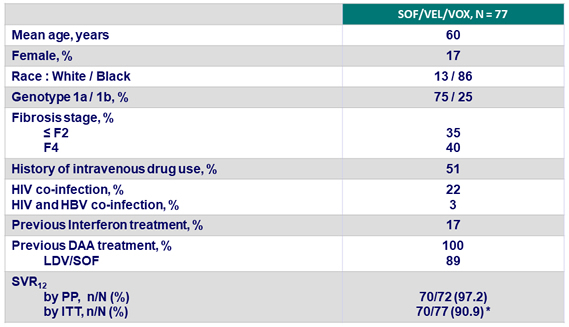
* 3 discontinuations for non-related adverse events, 2 lost to follow-up, 1 death (HCC), 1 relapse
Adverse events
- Adverse events related to study drug in ≥ 5%
- Fatigue: 27%
- Headache: 24%
- Diarrhea: 21%
- Abdominal pain: 9%
- Nausea: 9%
- Constipation: 6%