EXPEDITION-8 Study: glecaprevir/pibrentasvir 8 weeks in patients with cirrhosis
Brown RS. AASLD 2018, Abs. LB-7
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1a
1b
1a
1b
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
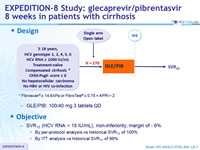
Design
* Fibroscan® ≥ 14.6 kPa or FibroTest® ≥ 0.75 + APRI > 2
- GLE/PIB: 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA < 15 IU/ml), non-inferiority, margin of - 6%
- By per-protocol analysis vs historical SVR12 of 100%
- By ITT analysis vs historical SVR12 of 99%
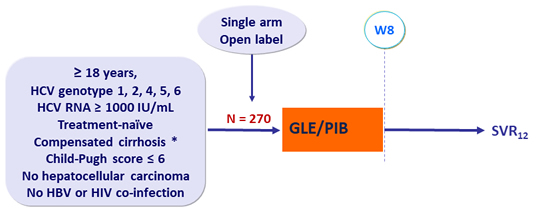
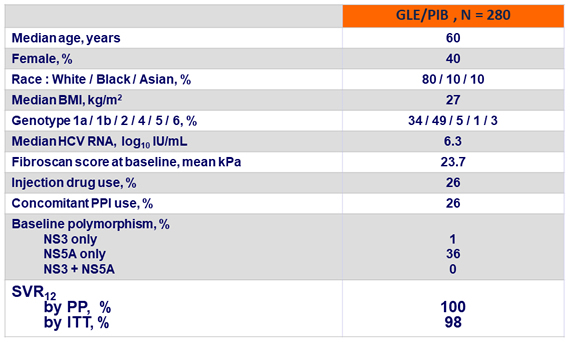
Baseline characteristics and SVR12
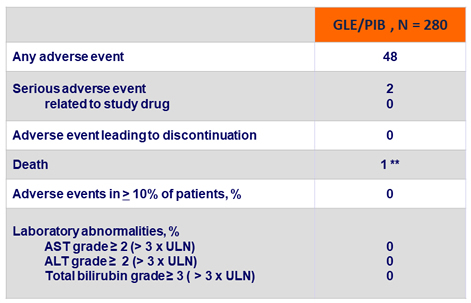
Adverse events and laboratory abnormalities, %
Summary
- GLE/PIB (300 mg/120 mg QD) for 8 weeks achieved high efficacy in patients with genotype 1, 2, 4, 6 and 6 HCV infection and compensated cirrhosis
- Non inferior to SVR12 in patients without cirrhosis
- High efficacy regardless of baseline characteristics
- No virologic failure
- GLE/PIB was well tolerated with a favorable safety profile
- Enrollment of patients with genotype 3 infection is ongoing