Glecaprevir/Pibrentasvir in genotype 1 with failure to NS5A-inhibitor plus sofosbuvir
Sulkowski MS. AASLD 2018, Abs. 0226
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
1a
1b
1
1a
1b
Treatment history
NS5A experienced
SOF-experienced
NS5A experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
Design
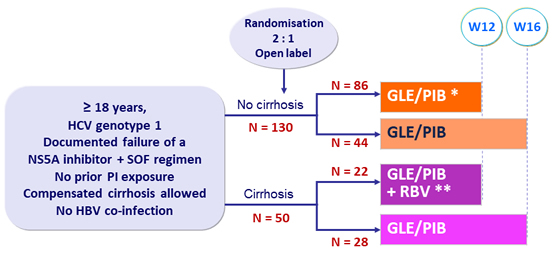
* 5 and ** 1 PI-experienced patients randomized to 12 weeks were treated for 16 weeks and analyzed with 16-W groups
- GLE/PIB: 100/40 mg 3 tablets QD
- Weight-based RBV
Objective
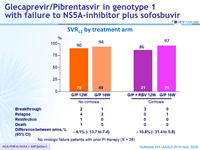
- SVR12 (HCV RNA < LLOQ)
- Comparison between treatment arms, cirrhosis vs no cirrhosis
- Comparison between treatment arms, 12 weeks vs 16 weeks treatment durations
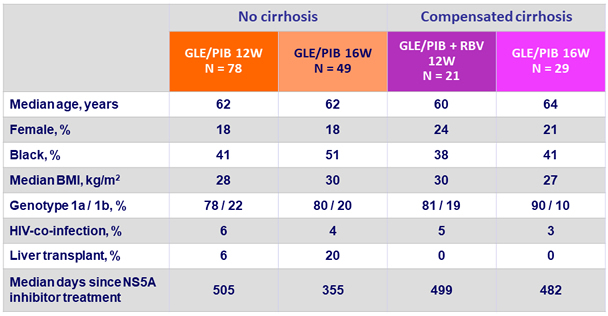
Baseline characteristics
SRV12 by treatment arm

- No virologic failure patients with prior PI therapy (N = 38)
SRV12 by G/P duration and genotype 1 subtype
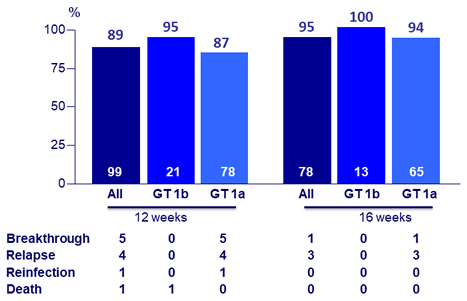
- No virologic failure in genotype 1b (N = 34)
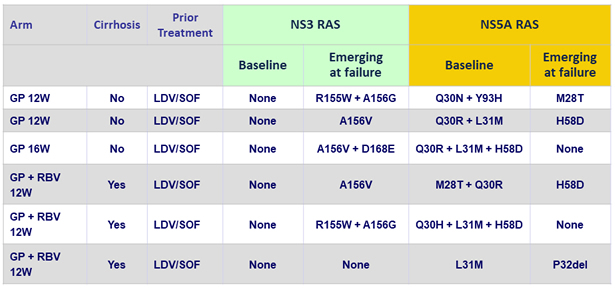
Virologic breakthrough (N = 6)
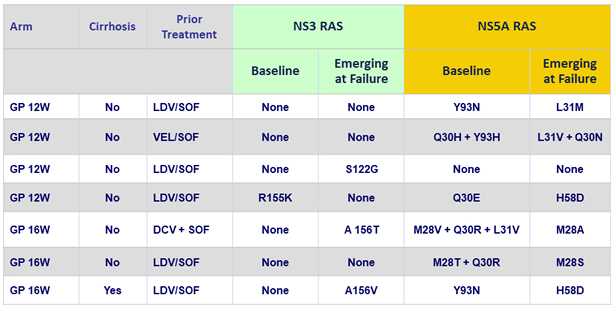
Relapse (N = 7)
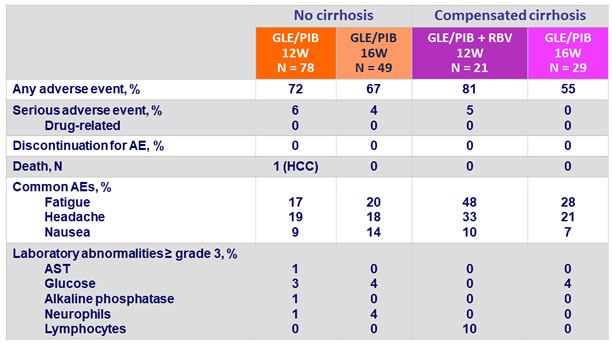
Adverse events and laboratory abnormalities