CORAL-I Study: OBV/PTV/r + DSV + RBV in liver transplantation with recurrent genotype 1 infection
An Interferon-free Antiviral Regimen for HCV after Liver Transplantation
Kwo PY. NEJM 2014;371:2375-82
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1a
1a
Special population
Liver transplantation
Liver transplantation
Design
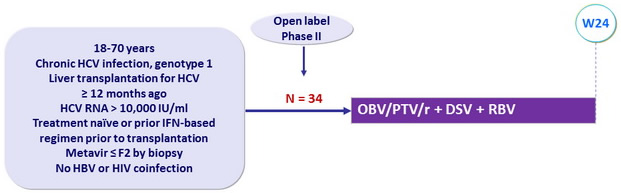
Stable immunosuppressive therapy : t acrolimus or cyclosporin ; glucocorticoïds at dose = 5 mg/day permitted
Treatment regimens
- Co-formulated ombitasvir / paritaprevir / rironavir / (OBV/PTV/r/): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
- RBV : dose selected by investigator ( most often 600-800 mg/ day )
Objective
- SVR12 , by intention to treat, with 95% CI, descriptive analysis
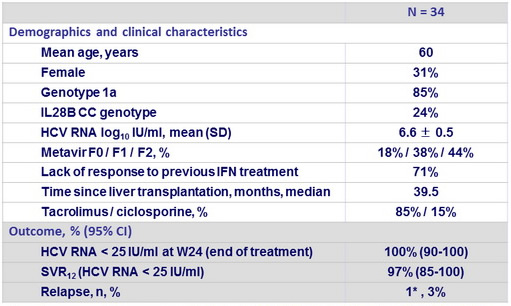
Baseline characteristics and treatment response
* At relapse : resistance-associated variants R155K in NS3, M28T and Q30R in NS5A, and G554S in NS5B
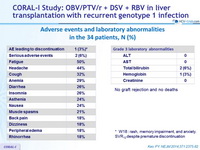
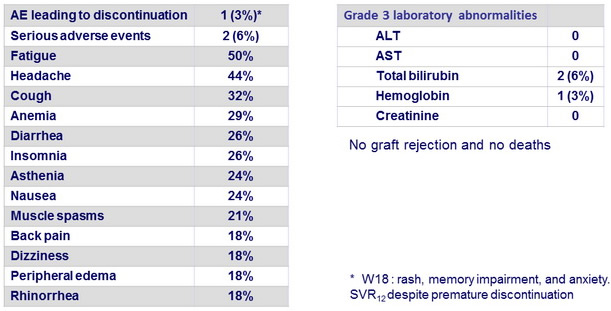
Adverse events and laboratory abnormalities in the 34 patients, N (%)
Summary
- In this phase II trial of a 24-week, IFN- free, all-oral antiviral regimen for HCV genotype 1 infection, a rate of sustained virologic response of 97% (95% CI , 85 to 100) at post-treatment weeks 12 and 24 was observed among liver-transplant recipients with no fibrosis or mild fibrosis.
- No deaths or episodes of graft rejection
- Very good tolerability and safety
- No patient needed a blood transfusion ; 5 patients (15%) required erythropoietin, all of whom had initially received RBV at a total daily dose of 1000 or 1200 mg
- Given the high SVR12 , regardless of the initial RBV dose , an initial dose of 600 to 800 mg may provide sufficient therapeutic benefit and minimize the risk of severe anemia
- Limitations
- Patients with advanced fibrosis excluded
- Patients with aggressive forms of recurrent HCV infection (e.g., fibrosing cholestatic hepatitis) excluded