COSMOS Study: SOF + SMV ± RBV for genotype 1 - Phase Iia
Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study
Lawitz E. Lancet 2014;384:1756-65
Anti-HCV
Sofosbuvir
Simeprevir
Ribavirin
Sofosbuvir
Simeprevir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
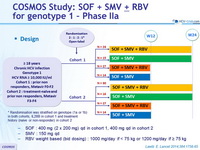
Design
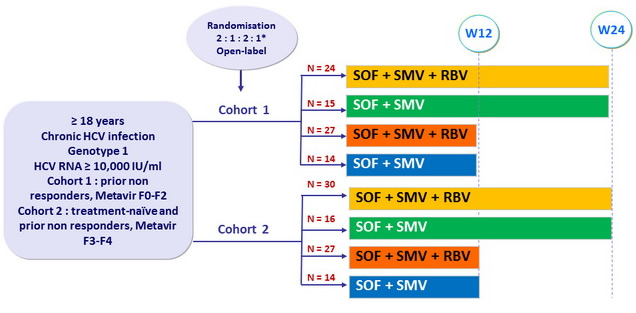
* Randomisation was stratified on genotype (1a or 1b) in both cohorts, IL28B in cohort 1 and treatment history (naïve or non-responder) in cohort 2
- SOF : 400 mg (2 x 200 mg) qd in cohort 1, 400 mg qd in cohort 2
- SMV : 150 mg qd
- RBV weight based (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if = 75 kg
Objective
- Exploratory study
- SVR12 (HCV RNA < 25 IU/ml), with 95% CI
- ITT analysis
- No sample size calculation
- Analyses within each cohort and on pooled cohort data
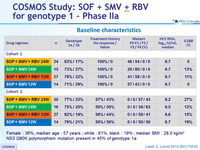
Baseline characteristics
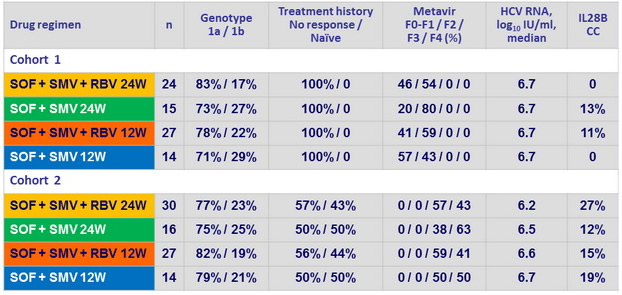
Female : 36%, median age : 57 years ; white : 81%, black : 19% ; median BMI : 28.0 kg/m 2
NS3 Q80K polymorphism mutation present in 45% of genotype 1a
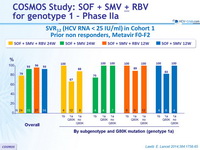
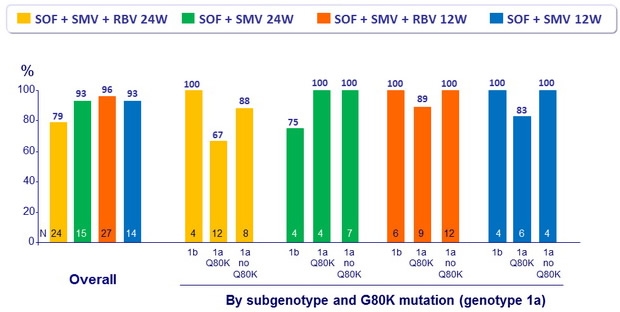
SVR12 (HCV RNA < 25 IU/ml) in Cohort 1
Prior non responders, Metavir F0-F2
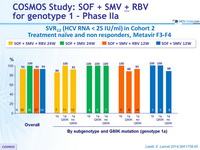
SVR12 (HCV RNA < 25 IU/ml) in Cohort 2
Treatment naïve and non responders, Metavir F3-F4
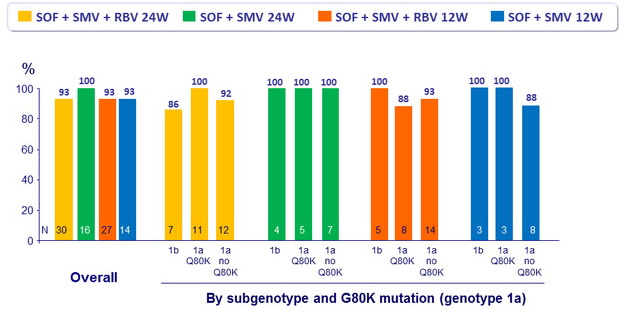
- SVR12 similar in patients with IL28B CC and non-CC genotypes, irrespective of whether they received RBV, in treatment-naïve or previous non-responders, with 12 or 24 weeks of SMF + SOF, also high in F4 patients
- Relapse in patients with HCV RNA < 25 IU /ml at end of completed treatment : 6 (all genotype 1a)
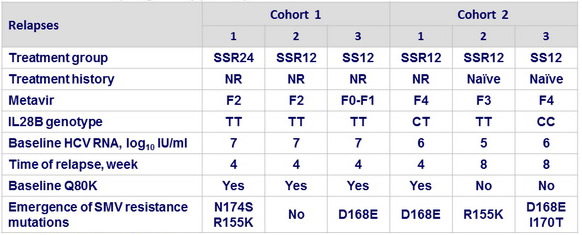
SSR : SOF + SMV + RBV ; NR : null responder
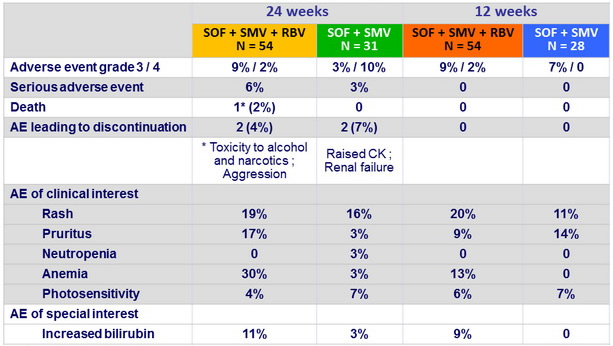
Adverse events , N (%)
Summary
- High Rates of SVR12 with SOF + SMV, even in difficult to treat groups, including patients with compensated cirrhosis and prior non response to therapy
- Rapid virologic response did not predict SVR12
- Addition of RBV did not improve SVR12 rates
- Extending treatment to 24 weeks did not improve SVR12 rates, except possibly in patients with prior relapse and advanced fibrosis
- Patients with baseline G80K mutation had high SVR12 rates, including those with compensated cirrhosis, with no impact of RBV
- Regimen well tolerated: fatigue, headache, and nausea were the most common side effects
- Overall, SOF + SMV shows high efficacy and tolerability in patients with chronic HCV genotype 1 infection, either naïve or non-responders to previous IFN-based therapy