Egyptian Ancestry Study: SOF + RBV for HCV genotype 4
Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry
Ruane P. J. Hepatology 2015;62:1040-6
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
4
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
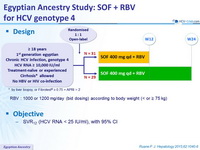
Design
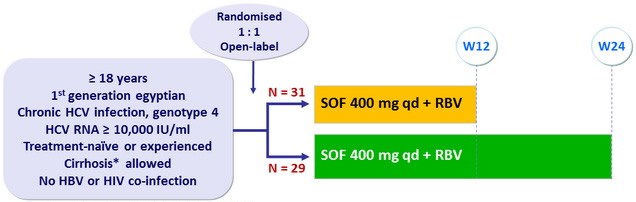
* by liver biopsy, or Fibrotest® = 0.75 + APRI > 2
RBV : 1000 or 1200 mg/ day ( bid dosing ) according to body weight (< or = 75 kg)
Objective
- SVR12 (HCV RNA < 25 IU/ml), with 95% CI
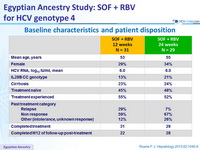
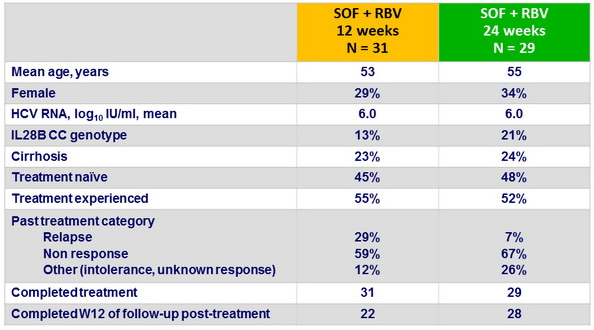
Baseline characteristics and patient disposition
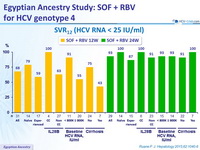
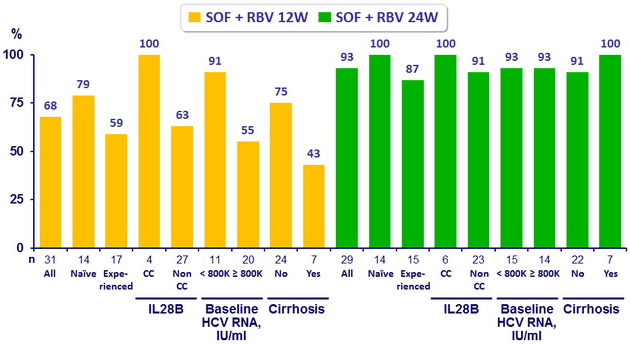
SVR12 (HCV RNA < 25 IU/ml)
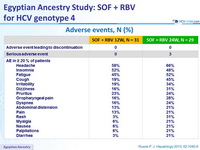
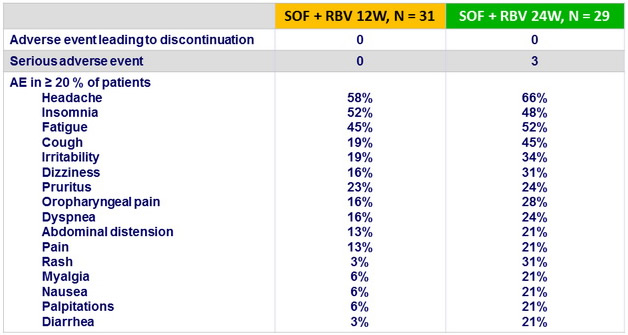
Adverse events, N (%)
Summary
- In this phase II, open-label study, 24 weeks of treatment with SOF and RBV resulted in high rates of SVR12 in treatment-naive and previously treated patients with genotype 4 HCV infection.
- SVR12 rates were notably high in patients with characteristics historically associated with poor response : cirrhosis, high baseline viral load, non-CC IL28B genotype, and prior non-response to HCV treatment
- Limitations
- Small sample size
- Small number (12 = 20%) of patients infected with non-4a HCV