FUSION Study: SOF + RBV (12 vs 16 weeks) for HCV genotypes 2 and 3
Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options
Jacobson IM. NEJM 2013;368:1867-77
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
2
3
2
3
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
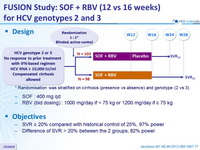
Design
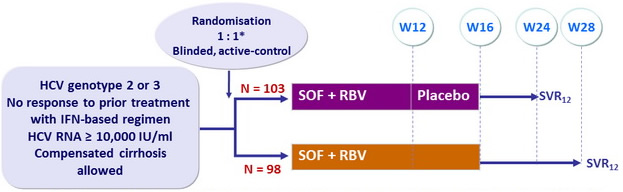
*
Randomisation was stratified on cirrhosis (presence vs absence) and genotype (2 vs 3)
- SOF : 400 mg qd
- RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if = 75 kg
Objective
- SVR = 20% compared with historical control of 25%, 97% power
- Difference of SVR > 20% between the 2 groups, 82% power
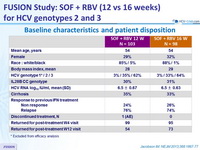
Baseline characteristics and patient disposition
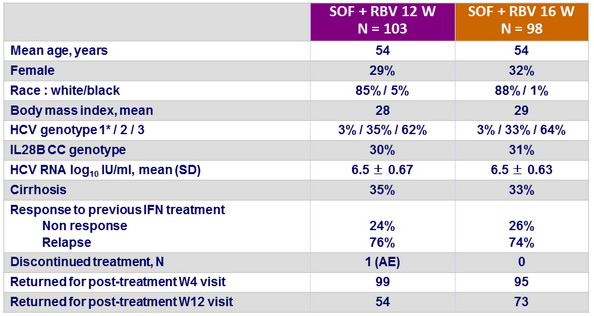
* Excluded from efficacy analysis
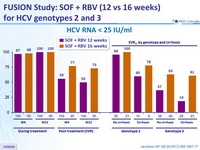
HCV RNA < 25 IU/ml
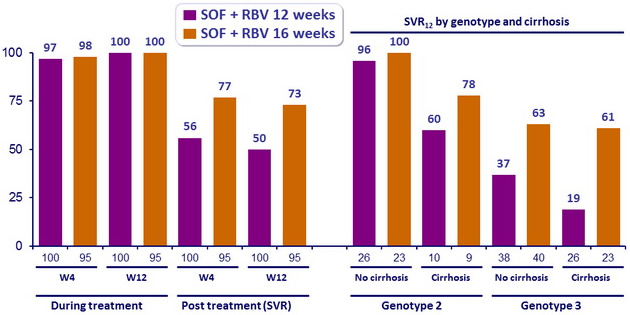
Virologic breakthrough during treatment
- none
Relapse in patients with HCV RNA < 25 IU/ml at end of completed treatment
- 46/99 (46%) in 12W group vs 26/95 (27%) in 16W group
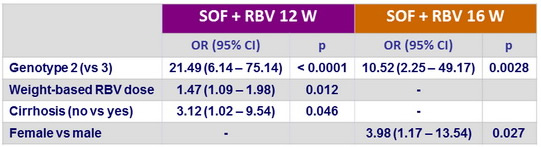
Multivariate analysis of factors associated with SVR12
Resistance testing (sequencing)
- 73 relapses :
- No SOF-associated mutation (S282T)
- 11 NS5B substitutions in > 2 subjects (no change in susceptibility to SOF)
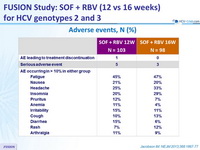
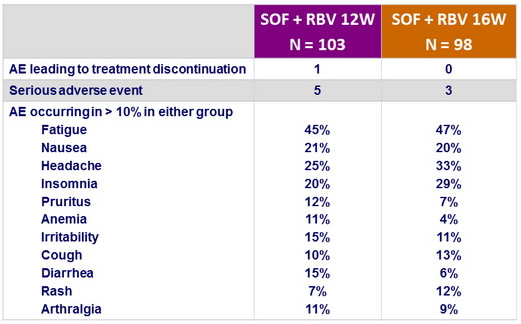
Adverse events , n (%)
Summary
- In this phase III study, 12 or 16 weeks of treatment with SOF and RBV resulted in a SVR12 in 50 to 73% of patients with prior treatment failure
- In genotype 2, h igh response rates (SVR12 > 96% ) were observed in patients with no cirrhosis ; those with cirrhosis had lower SVR12 (60 to 78%)
- In genotype 3 infection, response rates were sub-optimal, whether patients had or not cirrhosis
- No virologic resistance was detected in patients who did not have a sustained virologic response
- The rate of premature discontinuation of treatment with SOF and RBV due to adverse events was low (1%)
- In conclusion, 12 weeks of treatment with SOF and RBV can be an effective option for patients with HCV genotype 2 infection who failed prior IFN-based therapy, in the absence of cirrhosis