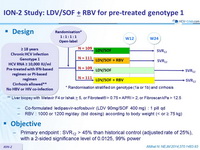
ION-2 Study: LDV/SOF ± RBV for pre-treated genotype 1
Ledipasvir and Sofosbuvir for Previously Treated HCV Genotype 1 Infection
Afdhal N. NEJM 2014;370:1483-93
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
IFN-Experienced
PI (NS3)-experienced
IFN-Experienced
PI (NS3)-experienced
Cirrhosis
Yes
No
Yes
No
Design
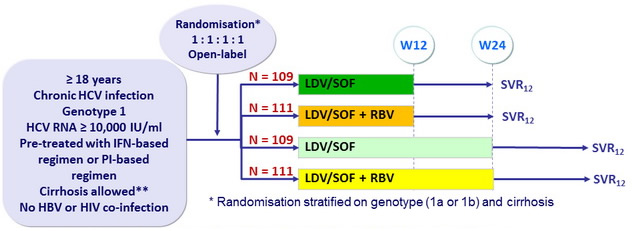
** Liver biopsy with Metavir F4 or Ishak > 5, or Fibrotest ® > 0.75 + APRI > 2, or Fibroscan kPa > 12.5
- Co-formulated ledipasvir-sofosbuvir (LDV 90mg/SOF 400 mg) : 1 pill qd
- RBV : 1000 or 1200 mg/ day ( bid dosing ) according to body weight (< or = 75 kg)
Objective
- Primary endpoint : SVR12 > 45% than historical control (adjusted rate of 25%), with a 2-sided significance level of 0.0125, 99% power
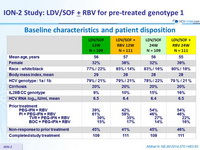
Baseline characteristics and patient disposition
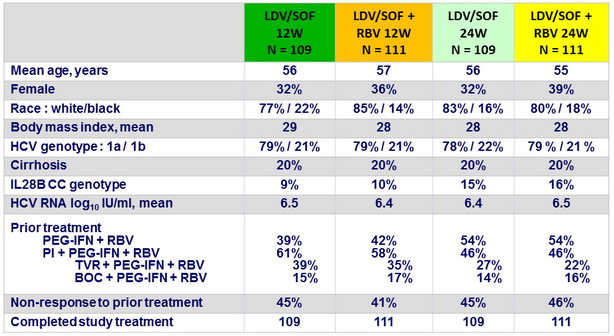
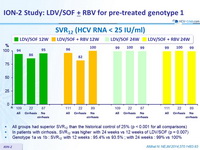
SVR12 (HCV RNA < 25 IU/ mL)
- All groups had superior SVR12 than the historical control of 25% (p < 0.001 for all comparisons)
- In patients with cirrhosis, SVR12 was higher with 24 weeks vs 12 weeks of LDV/SOF (p = 0.007)
- Genotype 1a vs 1b : SVR12 with 12 weeks : 95.4% vs 93.5% ; with 24 weeks : 99% vs 100 %
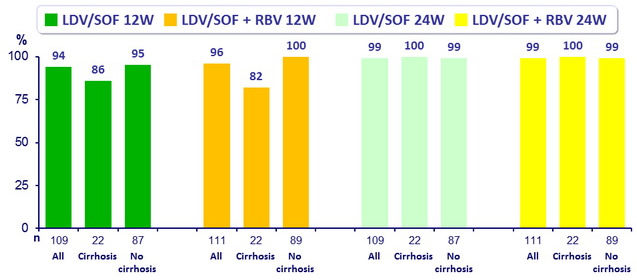
Virologic outcome - Mutivariate analysis of predictors of SVR12
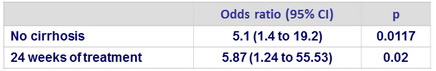
Virologic failure
- Virologic breaktrough : 1 in LDV/SOF + RBV 24W (non adherence)
- Post-treatment relapse
- 0 in the 24-weeek groups
- 7 (6%) in LDV/SOF 12W and 4 (4%) in LDV/SOF + RBV 12W
- NS5A resistant variants
(deep sequencing)
- Baseline resistance in 62 (14%) of 439 patients tested : SVR12 in 55/62 (89%)
- 6 of the 11 patients (55%) with relapse had baseline NS5A resistance
- All 11 patients had NS5A resistant variants at relapse
- Similar viral kinetics during early phase of treatment in patients with or without NS5A variants
- No S282T NS5B-resistant variants detected at baseline or after treatment
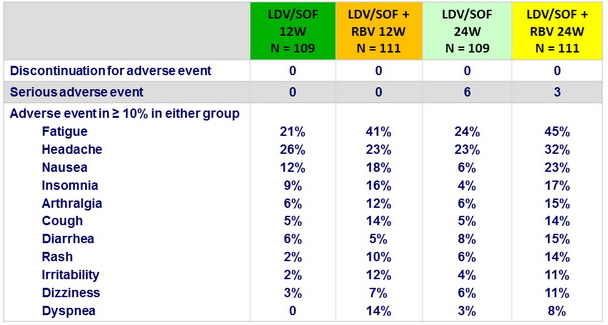
Adverse events
Summary
- 12 or 24 weeks of the single-tablet regimen of LDV/SOF was a highly effective treatment for patients with HCV genotype 1 infection who had not had a SVR after prior IFN-based treatment, including protease inhibitor
- Rates of SVR12 (94 to 99%) were similar with 12 or 24 weeks of treatment, with no additional benefit of RBV addition
- Rates of SVR12 were high (92 to 100%) in difficult-to-treat subgroups : IL28B non-CC, prior nonresponse to IFN-based regimen, including PI-based
- In the 12-week groups, lower rate of SVR12 in patients with cirrhosis
- LDV/SOF was not associated with any new or characteristic adverse events
- Rate of AE was lower in 12-week LDV/SOF group vs the 3 other groups
- Relapse occurred in 11 patients (5%) in the 12-week groups
- None had evidence of mutations conferring resistance to SOF
- Mutations associated with resistance to NS5A inhibitors were present in 6/11 patients at baseline and in all at the time of relapse