NEUTRINO Study: SOF + PEG-IFNα-2a + RBV
Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection
Lawitz E. NEJM 2013;368:1878-87
Anti-HCV
Sofosbuvir
PEG-IFNα 2a
Ribavirin
Sofosbuvir
PEG-IFNα 2a
Ribavirin
Genotype
1
1a
1b
4
1
1a
1b
4
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
Design
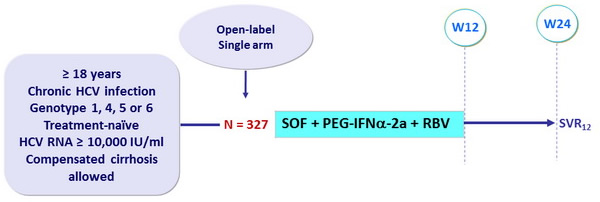
- SOF : 400 mg qd
- PEG-IFNα-2a : 180 mg SC once weekly
- RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if ≥ 75 kg
Objective
- SVR12 > 60%, 90% power
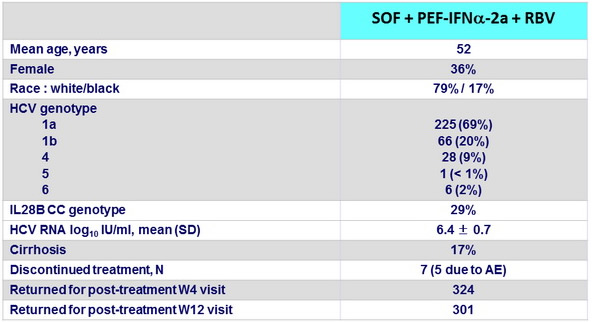
Baseline characteristics and patient disposition
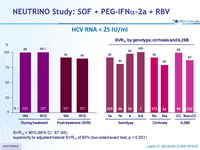
HCV RNA < 25 IU/ml
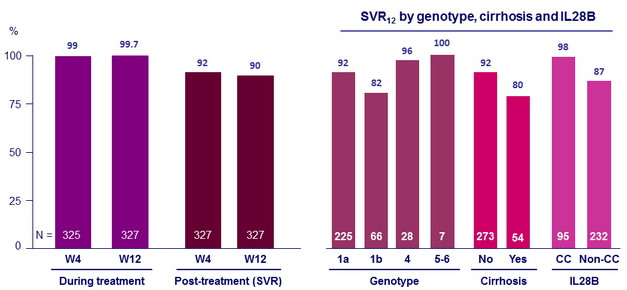
SVR12 = 90% (95% CI: 87-93) : superiority to adjusted historal SVR12 of 60% (two-sided exact test, p < 0.001)
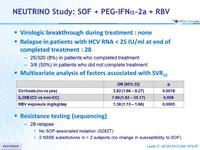
- Virologic breakthrough during treatment : none
- Relapse in patients with HCV RNA < 25 IU/ml at end of completed treatment : 28
- 25/320 (8%) in patients who completed treatment
- 3/6 (50%) in patients who did not complete treatment
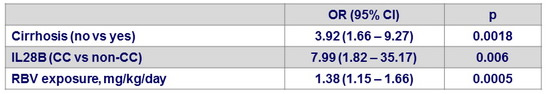
Multivariate analysis of factors associated with SVR12
- Resistance testing (sequencing) :
28 relapse
- No SOF-associated mutation (S282T)
- 2 NS5B substitutions in > 2 subjects (no change in susceptibility to SOF)
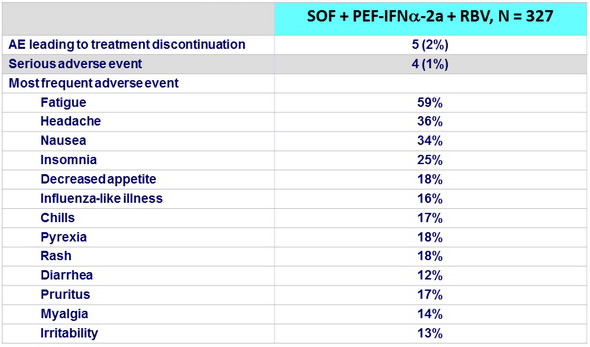
Adverse events , n (%)
Summary
- In this open-label, single-group study of SOF + PEG-IFNαnd RBV in previously untreated patients with HCV genotype 1 or 4 infection, a SVR of 90% at 12 weeks was obtained
- In patients with genotype 1 infection who had cirrhosis SVR12 was lower than for patients without cirrhosis (81% vs 92%)
- Patients with genotype 1, 4, 5, or 6 infection who received 12 weeks of SOF + PEG-IFN + RBV had a very low rate of treatment discontinuation (2%)
- No virologic resistance was detected in patients who did not have a sustained virologic response