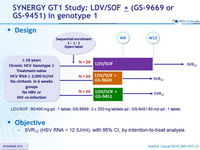
SYNERGY GT1 Study: LDV/SOF ± (GS-9669 or GS-9451) in genotype 1
Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study
Kohli A. Lancet 2015;385:1107-13
Anti-HCV
Ledipasvir
Sofosbuvir
Vedroprevir (GS-9451)
Radalbuvir (GS-9669)
Ledipasvir
Sofosbuvir
Vedroprevir (GS-9451)
Radalbuvir (GS-9669)
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
LDV/SOF : 90/400 mg qd : 1 tablet; GS-9669 : 2 x 250 mg tablets qd ; GS-9451 80 md qd : 1 tablet
Objective
- SVR12 (HSV RNA < 12 IU /ml) , with 95% CI, by intention-to-treat analysis
Baseline characteristics and outcome
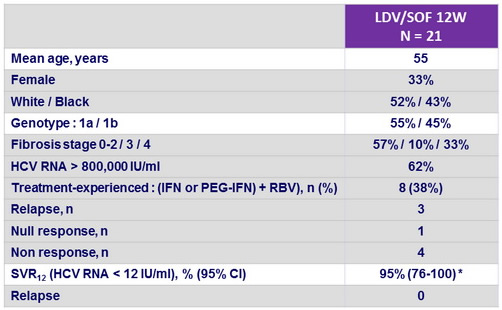
* Patient with relapse : genotype 1a, IL28B CT genotype, baseline HCV RNA 1 922 287 IU/ml, baseline NS5A resistance-associated variants : M28T + Q30H ;
at relapse : M28T + Q30T, no SOF resistance-variants, no GS-9669 resistance-variants
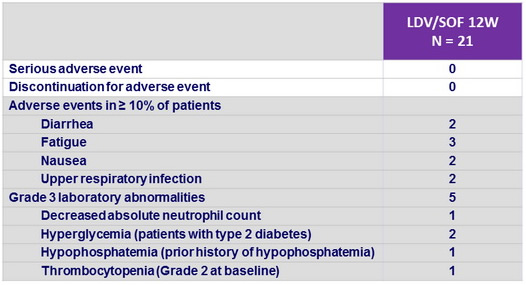
Adverse events, N
Summary
- Patients with chronic HCV genotype 1 infection without cirrhosis were successfully treated with a 6 week course of three oral direct-acting antiviral drugs
- The regimens were well tolerated, rapidly suppressed HCV viremia in patients, and resulted in high rates of SVR12
- Furthermore, viral kinetic modeling suggests that the 3-drug regimen of LDV/SOF + GS-9451 - targeting 3 different stages of the HCV lifecycle - resulted in enhanced HCV clearance compared with other regimens that target only two stages
- One patient treated for 6 weeks relapsed 2 weeks after treatment completion
- Limitations
- Small sample size
- Exclusion of patients with cirrhosis in the 6 weeks groups