NIH SPARE Study: SOF + RBV in genotype 1 with advanced liver disease
Sofosbuvir and Ribavirin for Hepatitis C Genotype 1 in Patients With Unfavorable Treatment Characteristics A Randomized Clinical Trial
Oisinusi A . JAMA 2013;310:804-11
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
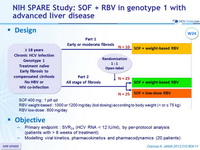
Design
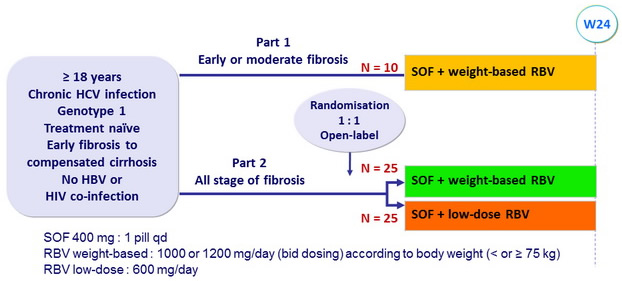
Objective
- Primary endpoint : SVR24 (HCV RNA < 12 IU/ml), by per-protocol analysis (patients with > 8 weeks of treatment)
- Modelling viral kinetics, pharmacokinetics and pharmacodynamics (20 patients)
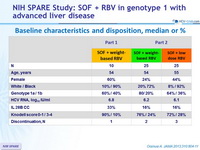
Baseline characteristics and disposition, median or %
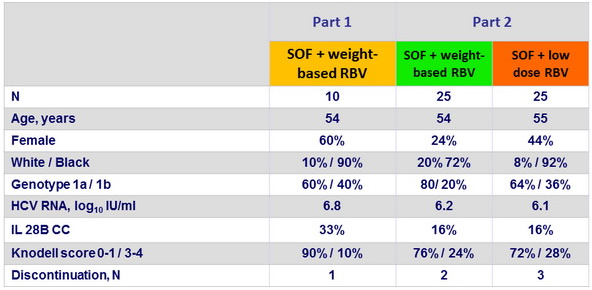
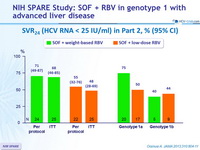
SVR24 (HCV RNA < 25 IU /ml) in Part 2, % (95% CI)
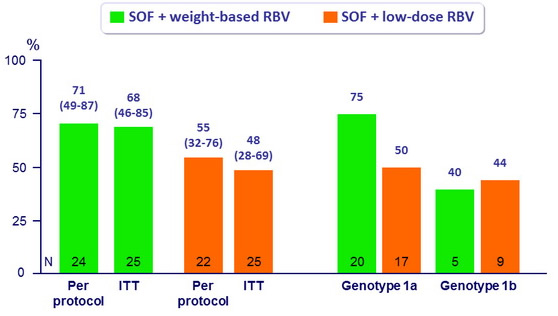
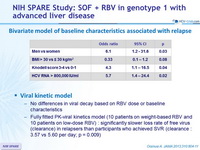
Bivariate model of baseline characteristics associated with relapse
Viral kinetic model
- No differences in viral decay based on RBV dose or baseline characteristics
- Fully fitted PK-viral kinetics model (10 patients on weight-based RBV and 10 patients on low-dose RBV) : significantly slower loss rate of free virus (clearance) in relapsers than participants who achieved SVR (clearance : 3.57 vs 5.60 per day; p = 0.009)
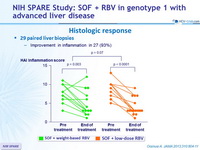
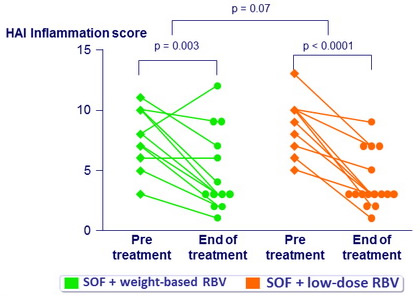
Histologic response
- 29 paired liver biopsies
- Improvement in inflammation in 27 (93%)
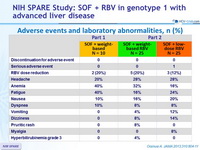
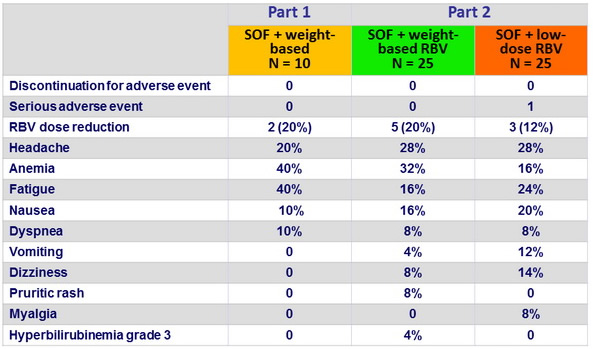
Adverse events and laboratory abnormalities, n (%)
Summary
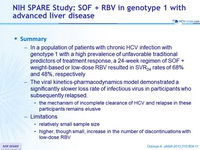
- In a population of patients with chronic HCV infection with genotype 1 with a high prevalence of unfavorable traditional predictors of treatment response, a 24-week regimen of SOF + weight-based or low-dose RBV resulted in SVR24 rates of 68% and 48%, respectively
- The viral kinetics-pharmacodynamics model demonstrated a significantly slower loss rate of infectious virus in participants who subsequently relapsed.
- the mechanism of incomplete clearance of HCV and relapse in these participants remains elusive
- Limitations
- relatively small sample size
- higher, though small, increase in the number of discontinuations with low-dose RBV