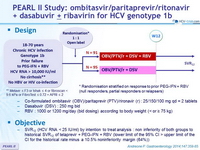
PEARL II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin for HCV genotype 1b
ABT-450, Ritonavir, Ombitasvir, and Dasabuvir Achieves 97% and 100% Sustained Virologic Response With or Without Ribavirin in Treatment-Experienced Patients With HCV Genotype 1b Infection
Andreone P. Gastroenterology 2014;147:359-65
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1b
1b
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
No
No
Design
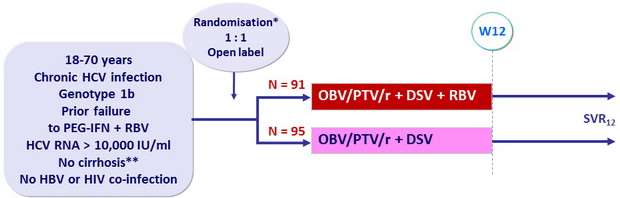
*
Randomisation stratified on response to prior PEG-IFN + RBV (null responders, partial responders or relapsers)
**
Metavir = F3 or Ishak = 4 or fibroscan < 9.6 kPa or FibroTest = 0.72 + APRI = 2
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r) : 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Objective
- SVR12 (HCV RNA < 25 IU /ml) by intention to treat analysis : non inferiority of both groups to historical SVR12 of telaprevir + PEG-IFN + RBV (lower limit of the 95% CI > upper limit of the CI for the historical rate minus a 10.5% noninferiority margin (64%))
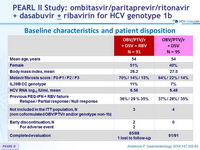
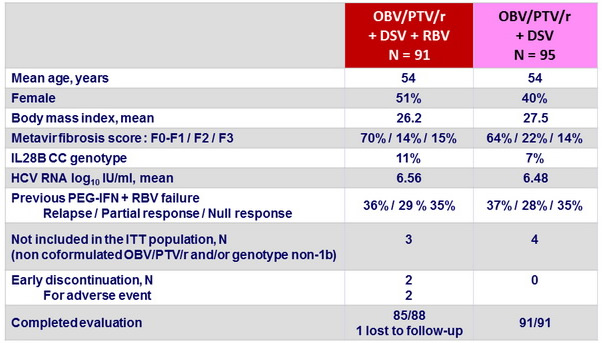
Baseline characteristics and patient disposition
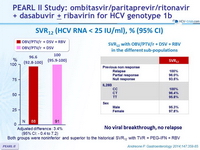
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
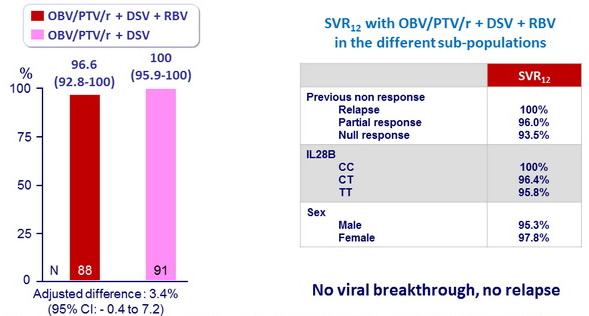
Both groups were noninferior and superior to the historical SVR12 with TVR + PEG-IFN + RBV
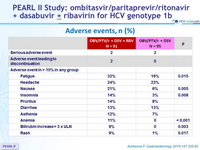
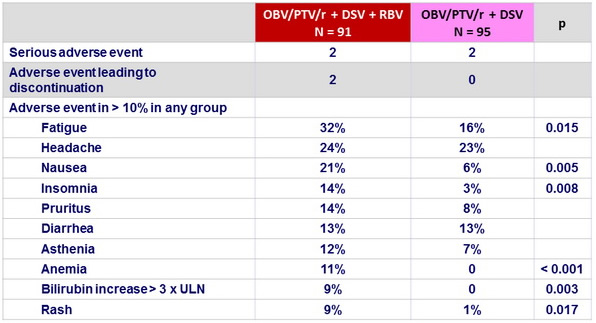
Adverse events, n (%)
Summary
- The ITT SVR12 rates in genotype 1b HCV infected patients receiving the 12-week regimen of OBV/PTV/r + DSV with or without RBV (96.6%–100%, respectively) were superior to the historical rate of telaprevir + PEG-IFN + RBV
- No benefit of addition of RBV, with significantly more adverse events, bilirubin increase and anemia
- Adverse events in both groups of 12-week regimens were generally mild and manageable.
- Overall, only 2 (1.1%) patients discontinued treatment because of adverse events, and the 5 serious adverse events reported in 4 patients were considered to be unrelated to study drug
- Limitations
- open-label study design
- exclusion of patients with cirrhosis, HBV or HIV co-infection