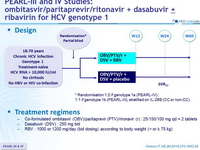
PEARL-III and IV Studies: ombitasvir / paritaprevir /ritonavir + dasabuvir ± ribavirin for HCV genotype 1
ABT-450/r–Ombitasvir and Dasabuvir with or without Ribavirin for HCV
Ferenci P. NEJM 2014;370:1983-92
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
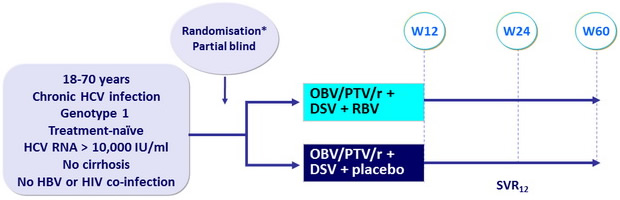
*
Randomisation 1:2 if genotype 1a (PEARL-IV) ; 1:1 if genotype 1b (PEARL-III), stratified on IL-28B (CC or non-CC)
Treatment regimens
- Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Efficacy endpoints
- Sustained virologic response (HCV RNA < 25 IU/mL) 12 weeks after end of treatment, with two-sided 95% CI, modified ITT analysis
- Non-inferiority of SVR assessed vs estimated rate of SVR24 with a telaprevir-based regimen
- for genotype 1a : 72%, 95% CI: 68 to 75
- for genotype 1b : 80%, 95% CI: 75 to 84
- Non-inferiority (primary end-point) established if lower bound of the 95% CI for the SVR greater than the upper bound of the 95% CI for SVR of telaprevir-based therapy minus 10.5%, i.e. 65% in PEARL-IV and 73% in PEARL-III ; power 95%
- Superiority in PEARL-IV (genotype 1a) if lower margin of the 95% CI for the SVR12 > 75% ; in PEARL-III (genotype 1b) if lower margin of the 95% CI for the SVR12 > 84% ; power 90%
- Non inferiority of RBV vs placebo in both studies : SVR12 rate, with lower margin of the 95% CI for the difference = -10.5% ; power 95%
Trial conduct
- Hemoglobin and hematocrit results blinded to investigators, unless criteria for virologic failure or relevant predefined toxicity were met
HCV RNA : COBAS TaqMan real-time RT–PCR assay, v 2.0 (Roche)
Virologic failure :
- 2 consecutive HCV RNA > 1 log10 IU/ml above the nadir at any time during treatment,
- HCV RNA ≥ 25 UI/ml at all assessments during treatment among patients who received at least 6 weeks of treatment,
- confirmed HCV RNA ≥ 25 IU/ml after a level < 25 IU/ml during treatment
Virologic relapse : confirmed HCV RNA ≥ 25 IU/ml between the end of treatment and 12 weeks after the last dose of study drug among patients who completed treatment and had an HCV RNA < 25 IU/ml at the final visit during the treatment period
Exploratory analysis : stepwise logistic-regression model to assess the association between the rate of SVR12 and continuous and categorical subgroup variables
Objective
- SVR12 (HCV RNA < 25 IU /ml) by intention to treat analysis : non inferiority of both groups to historical SVR12 of telaprevir + PEG-IFN + RBV (lower limit of the 95% CI > upper limit of the CI for the historical rate minus a 10.5% noninferiority margin (64%))
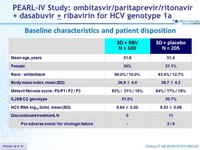
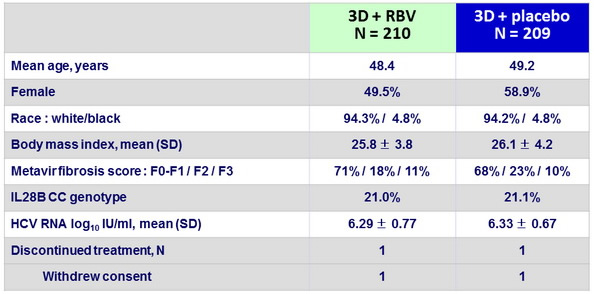
Baseline characteristics and patient disposition
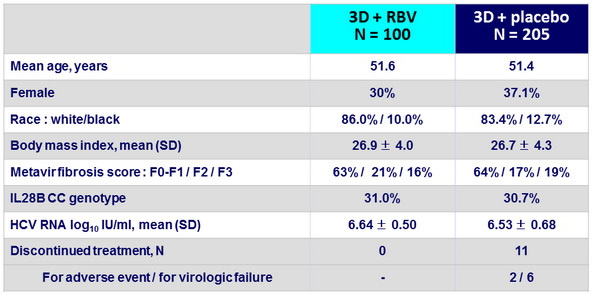
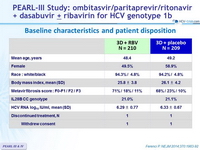
Baseline characteristics and patient disposition
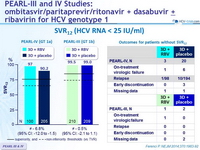
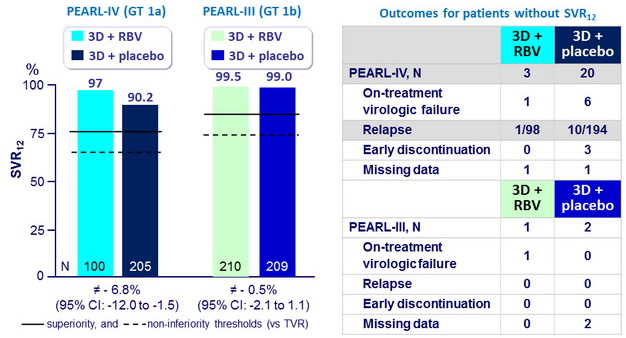
SVR12 (HCV RNA < 25 IU/ml)
Multivariate analysis of factors associated with increased SVR
- IL28B CC genotype (p = 0.03)
Resistance testing (population sequencing)
- 18 virologic failures (on-treatment or relapse)
- Resistance, N = 18/18
- Most frequent variants :
- D168V (NS3),
- M28T and Q30R (NS5A),
- S556G (NS5B)
- Most frequent variants :
- Resistance, N = 18/18
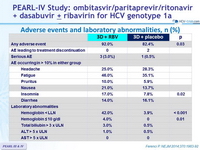
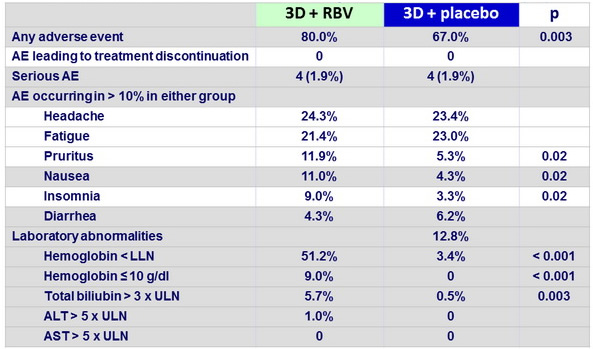
Adverse events and laboratory abnormalities, n (%)
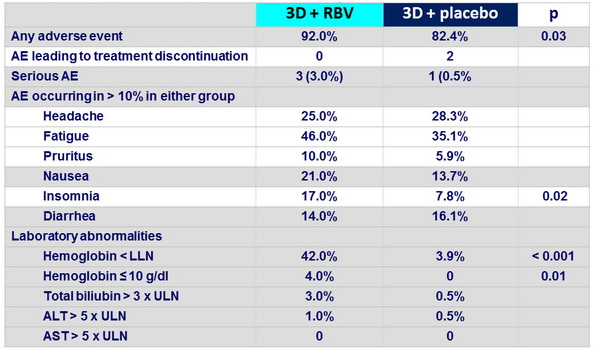
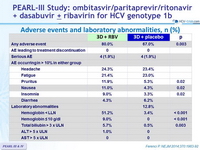
Adverse events and laboratory abnormalities, n (%)
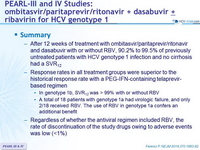
Summary
- After 12 weeks of treatment with ombitasvir/paritaprevir/ritonavir and dasabuvir with or without RBV, 90.2% to 99.5% of previously untreated patients with HCV genotype 1 infection and no cirrhosis had a SVR12
- Response rates in all treatment groups were superior to the historical response rate with a PEG-IFN-containing telaprevir-based regimen
- In genotype 1b, SVR12 was > 99% with or without RBV
- A total of 18 patients with genotype 1a had virologic failure, and only 2/18 received RBV. The use of RBV in genotype 1a confers an additional benefit
- Regardless of whether the antiviral regimen included RBV, the rate of discontinuation of the study drugs owing to adverse events was low (<1%)