ALLY-3 Study: DCV + SOF for HCV genotype 3
All-Oral 12-Week Treatment With Daclatasvir Plus Sofosbuvir in Patients With Hepatitis C Virus Genotype 3 Infection: ALLY-3 Phase III Study
Nelson DR. Hepatology 2015;61:1127-35
Anti-HCV
Daclatasvir
Sofosbuvir
Daclatasvir
Sofosbuvir
Genotype
3
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
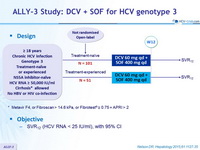
Design
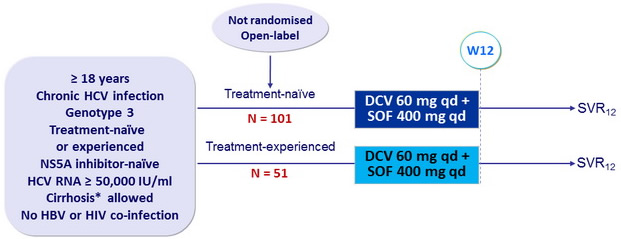
* Metavir F4, or Fibroscan > 14.6 kPa , or Fibrotest ® = 0.75 + APRI > 2
Objective
- SVR12 (HCV RNA < 25 IU/ml), with 95% CI
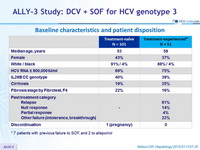
Baseline characteristics and patient disposition
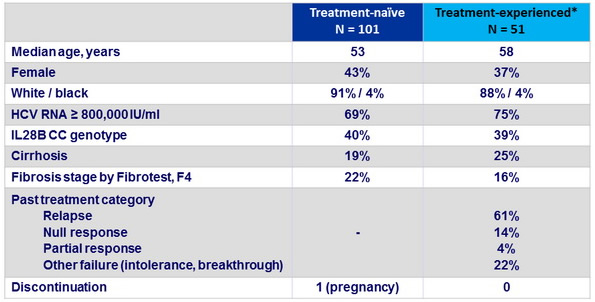
* 7 patients with previous failure to SOF, and 2 to alisporivir
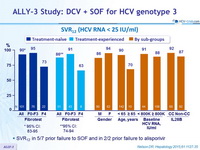
SVR12 (HCV RNA < 25 IU/ml)
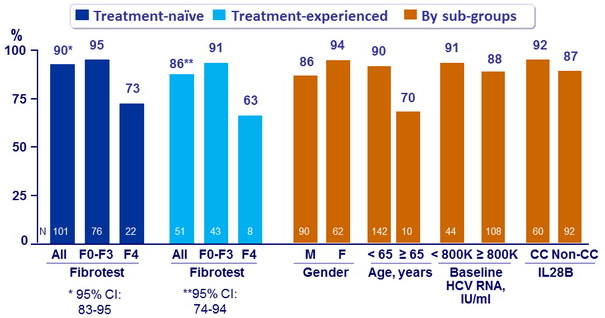
- SVR12 in 5/7 prior failure to SOF and in 2/2 prior failure to alisporivir
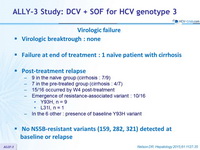
Virologic failure
- Virologic breaktrough : none
- Failure at end of treatment : 1 naïve patient with cirrhosis
- Post-treatment relapse
- 9 in the naïve group (cirrhosis : 7/9)
- 7 in the pre-treated group (cirrhosis : 4/7)
- 15/16 occurred by W4 post-treatment
- Emergence of resistance-associated variant : 10/16
- Y93H, n = 9
- L31I, n = 1
- In the 6 other : presence of baseline Y93H variant
- No NS5B-resistant variants (159, 282, 321) detected at baseline or relapse
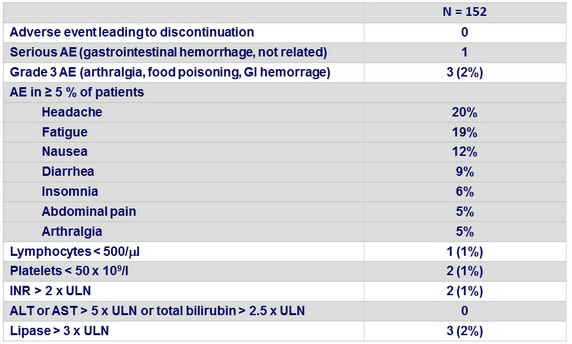
Adverse events and Grade 3-4 laboratory abnormalities, N (%)
Summary
- A 12-week regimen of DCV plus SOF achieved SVR12 in 96% of treatment-naïve and treatment-experienced patients with genotype 3 infection without cirrhosis and was well tolerated
- SVR in patients with cirrhosis was sub-optimal
- SVR12 rates were comparable across subgroups based on gender, age, baseline HCV-RNA levels, and IL28B genotype
- Among the 16 patients with relapse, 11 had cirrhosis
- 6/13 patients with baseline Y93H variant had relapse post-treatment, including 3 of 4 patients with cirrhosis
- Safety was good with no discontinuation for adverse event