SIRIUS Study: LDV/SOF ± RBV for genotype 1 and cirrhosis with non response to prior PI therapy
Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS)
Bourlière M. Lancet Infect Dis 2015;15:397-404
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1a
1b
1
1a
1b
Treatment history
PI (NS3)-experienced
PI (NS3)-experienced
Cirrhosis
Yes
Yes
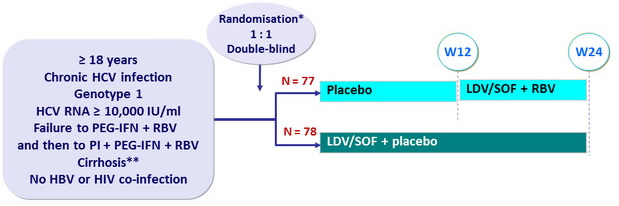
Design
*
Randomisation stratified on genotype (1a or 1b) and response to previous treatment (HCV RNA < lower limit of quantification acheived or not achieved)
** Liver biopsy or Fibroscan > 12.5 kPa or Fibrotest® > 0.75 + APRI > 2
- Co-formulated ledipasvir-sofosbuvir (LDV 90mg/SOF 400 mg) : 1 pill qd
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Objective
- SVR12 (HCV RNA < 25 IU /ml) , with two sided 95% CI : difference of 15% between groups, 80% power
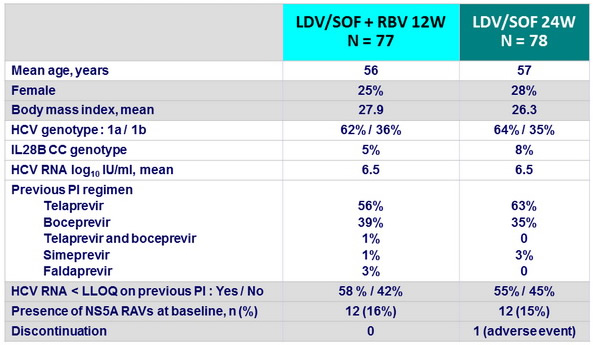
Baseline characteristics and patient disposition
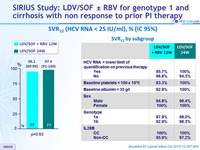
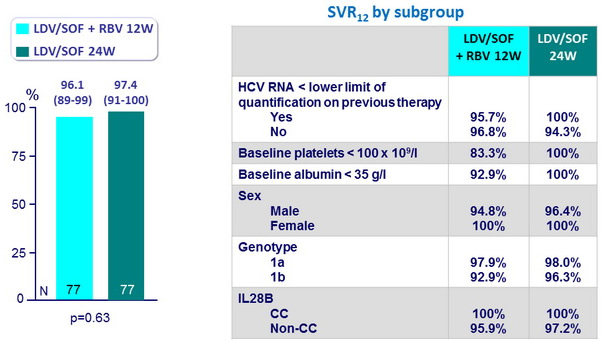
SVR12 (HCV RNA < 25 IU/ml), % (IC 95%)
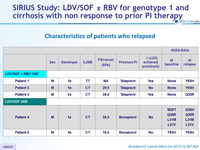
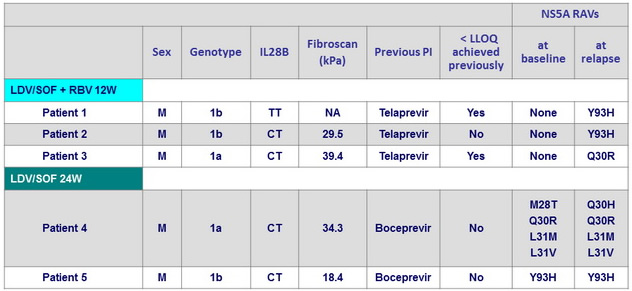
Characteristics of patients who relapsed
Adverse events
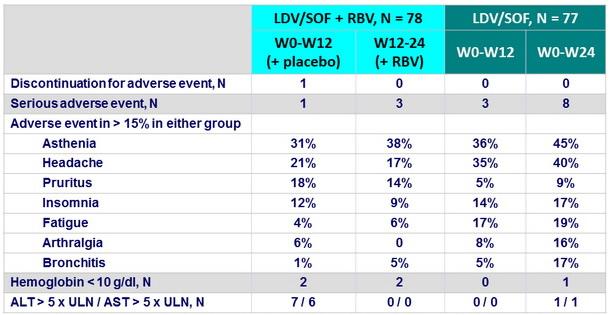
Other adverse events occurring in 5-15% of patients : nausea, cough, diarrhea, irritability, myalgia, dry skin, back pain, sleep disorder, dyspnea
Summary
- In this randomised, phase II study, patients with HCV genotype 1 infection and compensated cirrhosis who had not previously achieved SVR with standard treatment achieved high SVR12 rates (96-97%) after treatment with LDV/SOF + RBV for 12 weeks or LDV/SOF for 24 weeks, with no statistical differences in rates between groups
- They were 5 relapses, and no baseline characteristics were predictive
- The 2 treatment regimens were safe and well tolerated
- Patients receiving LDV/SOF alone had higher rates of headache and fatigue than patients receiving LDV/SOF + RBV or placebo
- Limitation
- Exclusion of patients with decompensated liver disease