SOF Failure Study: LDV/SOF + RBV for genotype 1 and prior failure to SOF
Ledipasvir-Sofosbuvir Plus Ribavirin for Patients With Genotype 1 Hepatitis C Virus Previously Treated in Clinical Trials of Sofosbuvir Regimens
Wyles D. Hepatology 2015; 61:1793-7
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
SOF-experienced
SOF-experienced
Design
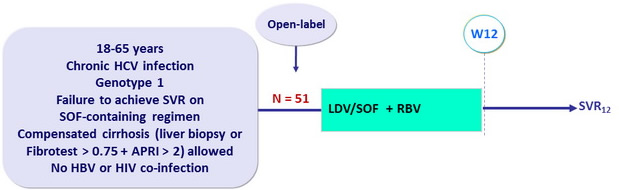
Treatment regimens
- LDV/SOF 90mg/400 mg : 1 pill qd
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Objective
- Primary endpoint : SVR12 (HCV RNA < 15 IU/ml) by intention to treat, with 2-sided 95% CI, no statistical hypothesis
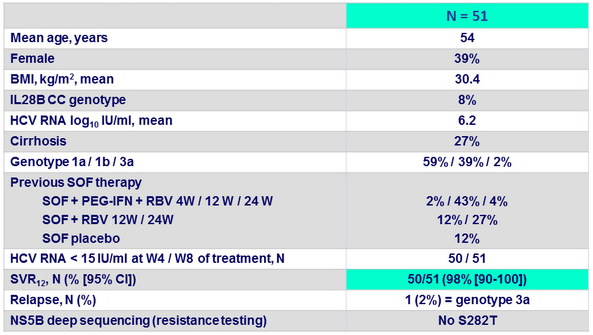
Baseline characteristics and outcome
Adverse events
- Serious adverse events : 4 in 2 patients
- Discontinuation due to adverse event in 1 patient, because of bipolar disorder
- Most common adverse events (≥ 10%) :
- Fatigue (25%)
- Headache (22%)
- Diarrhea (14%)
- Insomnia (12%)
- Rash (12%)
- Nausea (10%)
- Decrease of hemoglobin < 10 g/dl in 2 patients
- Grade 4 hyperbilirubinemia in 1 patient
Summary
- In this open-label study, all patients with genotype 1 HCV who had previously failed SOF + RBV or SOF + PEG-IFN + RBV achieved SVR after 12 weeks of treatment with the fixed-dose combination of LDV/SOF + RBV
- The only patient who did not achieve SVR was a patient with genotype 3 who had been mistakenly enrolled




