Japanese SOF + RBV Study: SOF + RBV in genotype 2
Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial
Omata M. J Viral Hepatitis 2015;21:762-8
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
2
2
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Design
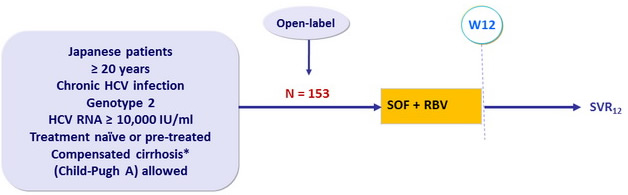
* Metavir = 4 or Ishak ≥ 5 or Fibroscan > 12.5 kPa
Treatment regimens
- SOF 400 mg : 1 pill qd
- RBV (bid dosing) : 600 mg/day if < 60 kg ; 800 mg/day if 60-80 kg ; 1000 mg/day if > 80 kg
Objective
- Primary endpoint : SVR12 (HCV RNA < 25 IU/ml) > 18% of historical SVR (69%) in naïve patients without cirrhosis, significance level of 0.05, 80% power
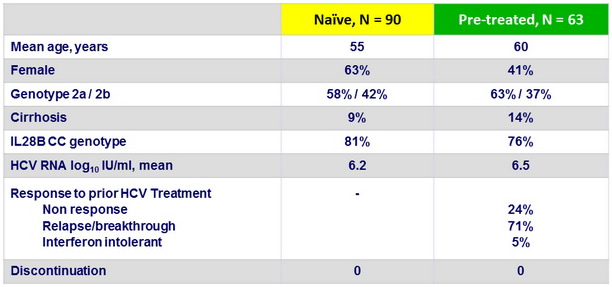
Baseline characteristics and patient disposition
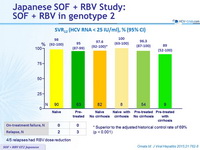
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
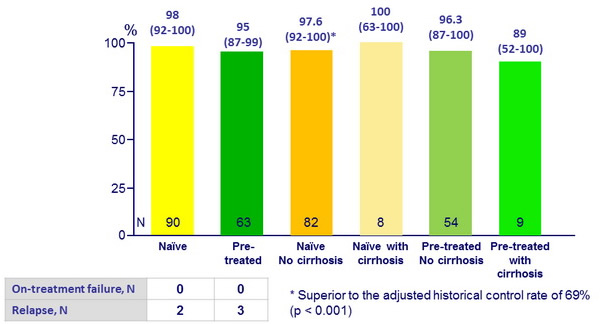
4/5 relapses had RBV dose reduction
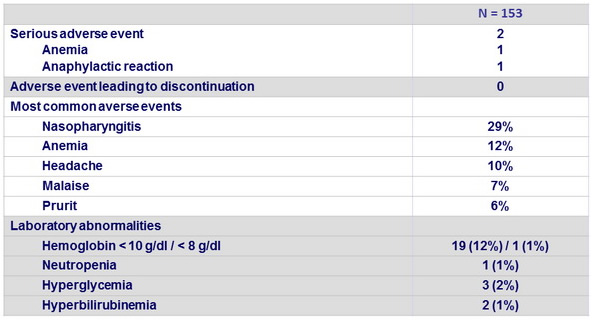
Adverse events, n (%)
Summary
- 12 weeks of treatment with SOF + RBV resulted in high rates of SVR12 (> 95%) in treatment-naïve and previously treated Japanese patients with chronic genotype 2 HCV infection
- In the present study, 22% of patients were aged 65 or older and 11% had cirrhosis
- Efficacy similar
- Increases in reported adverse events and laboratory abnormalities in patients ≥ 65 years, but these differences did not present a barrier to treatment as no premature discontinuation of study treatment occurred in any patient
- Relapse rate was 3%, and none of the subjects who relapsed had S282T or other nucleoside inhibitor resistance-associated variants