SOLAR-1 Study: LDV/SOF + RBV in advanced liver disease
Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease
Charlton M. Gastroenterology 2015 ; 149: 649-659
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Cirrhosis
Yes
Yes
Special population
Liver transplantation
Liver transplantation
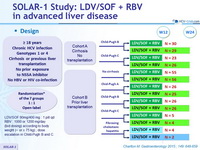
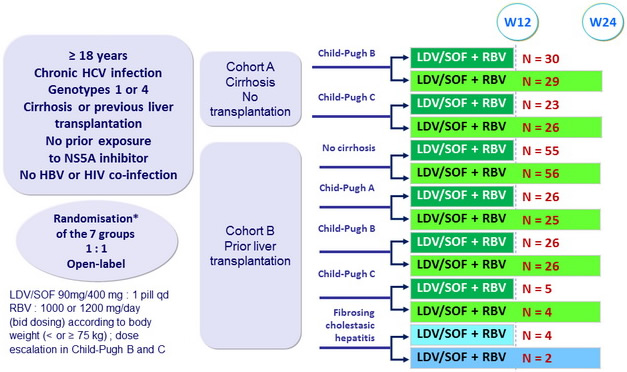
Design
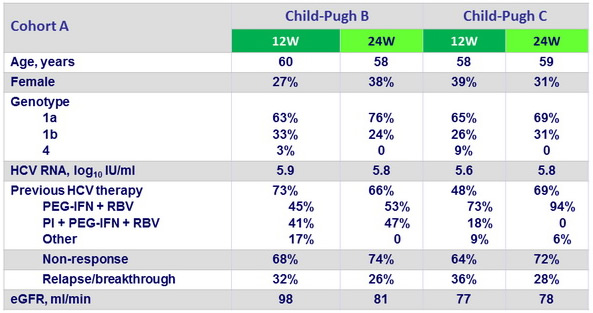
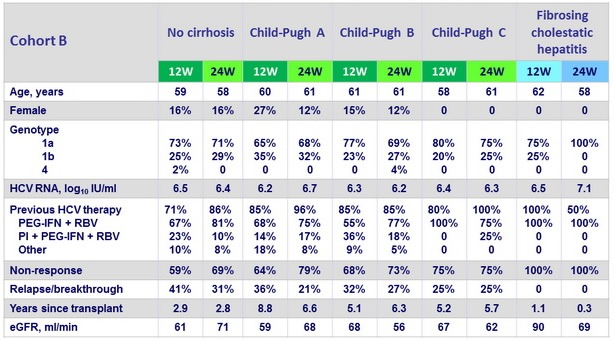
Baseline characteristics in Cohort A (cirrhosis, pre-transplantation), median or %
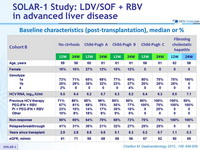
Baseline characteristics (post-transplantation), median or %
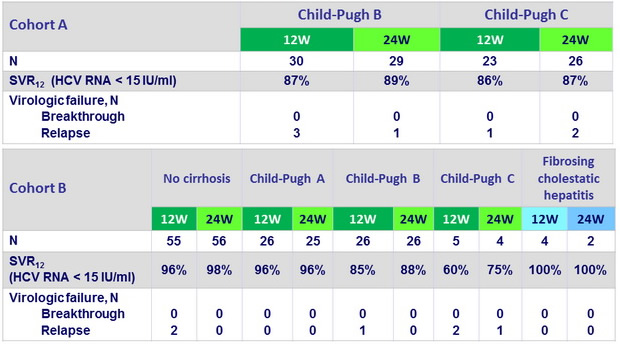
Virologic response
Virologic resistance
- Relapse in 7% (3/42) with baseline NS5A resistance-associated variants versus 4% (10/269) in patients without baseline NS5A RAVs
- No relapse in patients treated 24 weeks
- Of the 13 relapses,
- 11 (85%) had emergence of NS5A RAVs
- M28T
- Q30H/R
- H58D
- Y93H/C
- 11 (85%) had emergence of NS5A RAVs
Liver transplantation in Cohort A
- N = 7 (4 during study treatment and 3 after completing treatment)
Deaths, N = 13
- Cohort A, Child-Pugh B, N = 3 ; Child-Pugh C, N = 3
- Cohort B, Child-Pugh A, N = 2 ; Child-Pugh B, N = 5 ; Child-Pugh C, N = 0
Serious Adverse events, N = 77 (23%)
- 30 (28.3% in cohort A
- 47 (20.5%) in cohort B
Discontinuation for adverse event, N = 13 (4%)
- 5 in Cohort A
- 8 in cohort B
- Reasons for discontinuation : sepsis or infection (n = 3), acute renal failure (N = 2), gastric hemorrhage (N = 1), ALT + AST increase (N = 1), dyspnea (N = 1)
Summary
- LDV/SOF + RBV for 12 weeks is an effective treatment for patients with advanced liver disease, including those with decompensated liver function before and after liver transplantation.
- Extending treatment to 24 weeks was not associated with improved outcomes, although there were no relapses in these groups
- Rates of SVR12 were over 85% in every group of patients with Child-Pugh class B decompensated cirrhosis - in those who had and had not undergone liver transplantation-, as well as those who received 12 and 24 weeks of treatment.
- Similar response rates were observed in Child-Pugh class C patients who had not undergone liver transplantation
- Results are very encouraging for fibrosing cholestatic hepatitis which may now be a manageable complication of liver transplantation
- SVR12 in patients with decompensated cirrhosis was associated with early improvements in Child-Pugh and MELD scores
- Patients with decompensated liver disease experienced high frequencies of RBV hematotoxicity