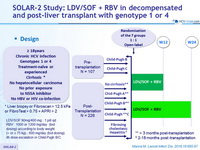
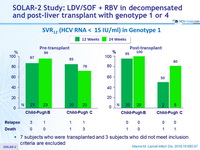
SOLAR-2 Study: LDV/SOF + RBV in decompensated and post-liver transplant with genotype 1 or 4
Ledipasvir/Sofosbuvir with Ribavirin for the Treatment of Fibrosing Cholestatic Hepatitis C after Liver Transplantation
Manns M. Lancet Infect Dis. 2016 ;16:685-97; Manns M. EASL 2015. Abs. GO2 ; Forns X. EASL 2015;Abs. P0779
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
4
1
1a
1b
4
Special population
Liver transplantation
Liver transplantation
Design
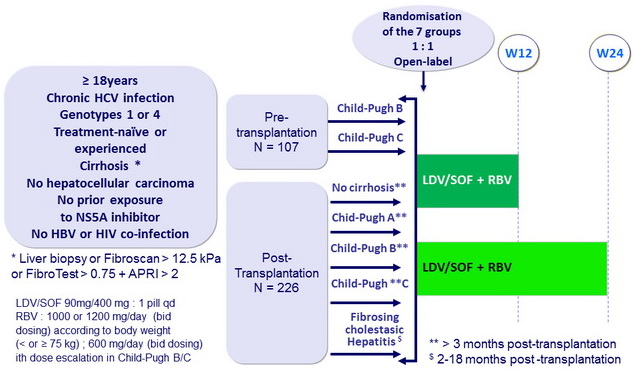
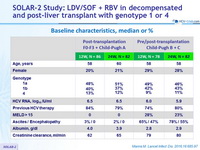
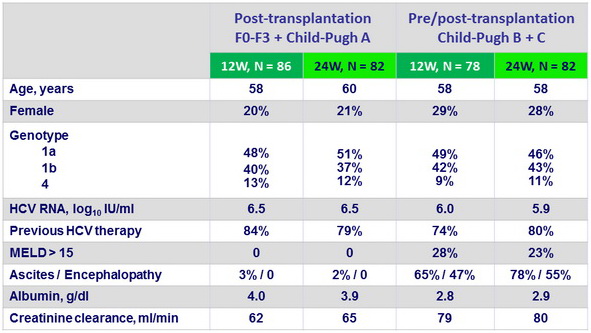
Baseline characteristics, median or %
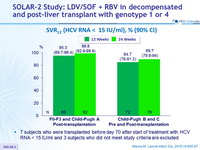
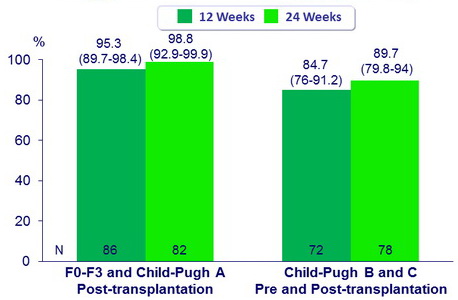
SVR12 (HCV RNA < 15 IU/ml), % (90% CI)
- 7 subjects who were transplanted before day 70 after start of treatment with HCV RNA < 15 IU/ml and 3 subjects who did not meet study criteria are excluded
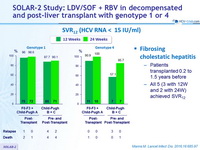
SVR12 (HCV RNA < 15 IU/ml)
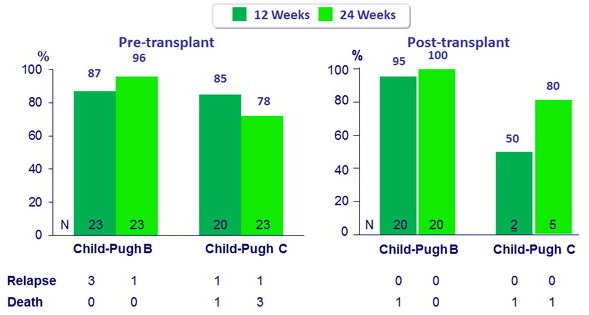
Fibrosing cholestatic hepatitis
- Patients transplanted 0.2 to 1.5 years before
- All 5 (3 with 12W and 2 with 24W) achieved SVR12
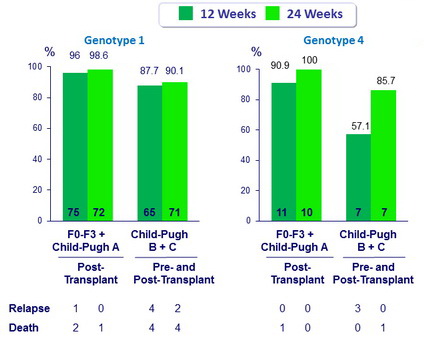
SVR12 (HCV RNA < 15 IU/ml) in Genotype 1
- 7 subjects who were transplanted and 3 subjects who did not meet inclusion criteria are excluded
Relapse and resistance
- Pretreatment resistance analysis using a 1% deep sequencing cutoff
- Genotype 1
- NS5A RAVs in 59/276 (21%) : 2/59 (3%) relapsed vs 5/217 (2.3%) with no RAVs (no relapse in the 32 patients with baseline NS5A RAVs with LDV/SOF + RBV 24W)
- Genotype 4
- NS5A RAVs in 24/32 (75%) : 3/24 relapsed vs 0/8 with no RAVs
- Genotype 1
- Overall, 10 relapses (decompensated cirrhosis in 9/10)
- Genotype 1 = 7 : genotype 1a = 5; genotype 1b = 2
- Genotype 4 = 3
- NS5A RAVs in genotype 1
- At pretreatment : 2/7 (positions Q30, L31, Y93I)
- At virologic failure : 7/7 (K24R, M28T, Q30H/K/T, L31M/V, Y93H/C)
- NS5A RAVs in genotype 4
- At virologic failure : 3/3 (L30H/R, M31V, P58L, Y93C)
- NS5B at relapse : 2 patients (genotype 1 and 4d) with E237G, no S282T
MELD score change from baseline to follow-up W12 in patients achieving SVR
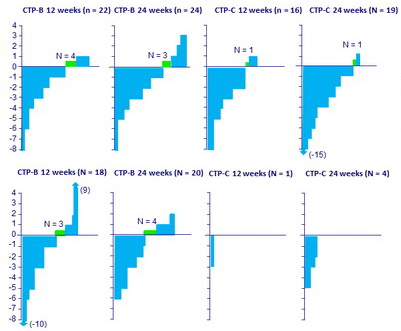
*Missing follow-up: N = 7
Improvements in MELD score:
- 58/81 (72%) of non-transplanted patients
- 25/43 (58%) of transplanted patients
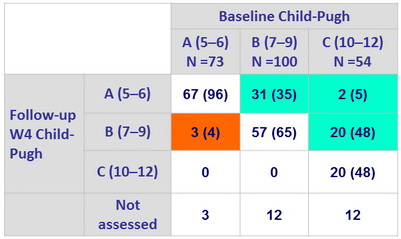
Liver function change from baseline to follow-up W4
Change in Child-Pugh Class, n (%)
Adverse events, n (%)
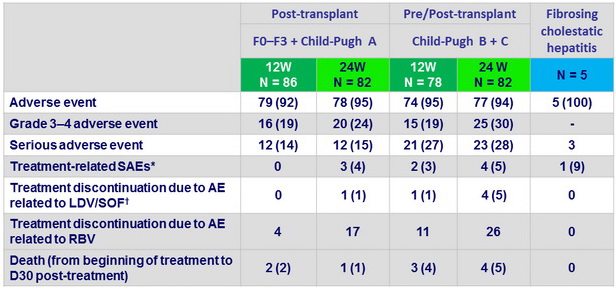
*Fall, anemia (5), vomiting, diarrhea, dyspnea, hyperbilirubinemia
†edema, dehydration, HCC (2), type 2 diabetes mellitus, hyperbilirubinemia
- No deaths were considered treatment related
Grade 3 or 4 laboratory abnormalities
- Most common : decreases in hemoglobin and lymphocytes, increases in total bilirubin and glucose
- Median creatinine concentrations remained stable in all groups
Ribavirin
- A verage daily dose
- Decompensated cirrhosis (transplanted or not) : 600 mg
- No cirrhosis or compensated cirrhosis : 800 mg
- Dose modification
- Reduction : 134 patients (40%)
- Interruption : 26 patients (8%)
- Discontinuation : 40 patients (12%)
- No association with relapse
Summary
- LDV/SOF + RBV resulted in high SVR12 rates in HCV patients with advanced liver disease, irrespective of transplantation status
- For genotype 1, SVR12 were similar between 12 and 24 weeks
- 12 weeks of treatment should become the standard of care for this group of patients
- Rates of relapse were low and were most frequently seen in non-transplanted patients with decompensated cirrhosis
- Similar SVR rates were recorded in patients with and without baseline NS5A resistance-associated variants whether using a 1% cutoff or 15% cutoff for both genotype 1 and genotype 4
- Among patients with cirrhosis, virologic response was associated with improvements in MELD and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (e.g. albumin)
- LDV/SOF + RBV for 12-24 weeks was generally safe and well tolerated in patients with advanced liver disease, pre and post liver transplantation
- Limitations
- Few genotype 4