TURQUOISE-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV in HIV co-infected patients
Ombitasvir, Paritaprevir Co-dosed With Ritonavir, Dasabuvir, and Ribavirin for Hepatitis C in Patients Co-infected With HIV-1 A Randomized Trial
Sulkowski MS, JAMA 2015;315:1223-31 ; Wyles D. J Infect Di 2017; 215:599-605
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1
1a
1
1a
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Special population
HIV co-infection
HIV co-infection
Design
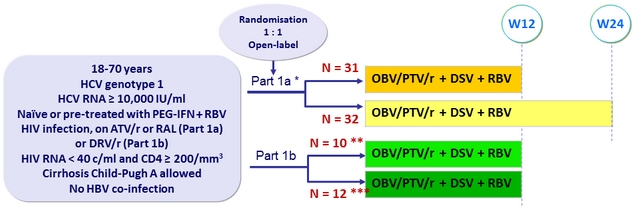
* Randomisation stratified on prior PEG-IFN + RBV therapy , and cirrhosis ; naïve patients also stratified on IL-28B (CC vs non-CC); experienced patients also stratified on prior response ( null , partial, relapse)
** DRV 800 mg qd ( ritonavir stopped ) ; *** DRV 600 mg bid + ritonavir 100 mg qd
Treatment regimens
- Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Primary efficacy endpoint
- SVR12 (HCV RNA < 25 IU/ml), with 2-sided 95% CI, comparison between groups
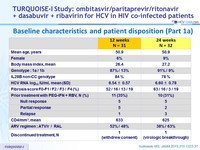
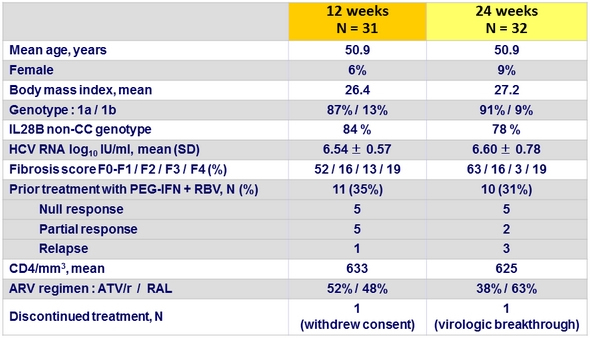
Baseline characteristics and patient disposition (Part 1a)
Virologic Outcome (Part 1a)
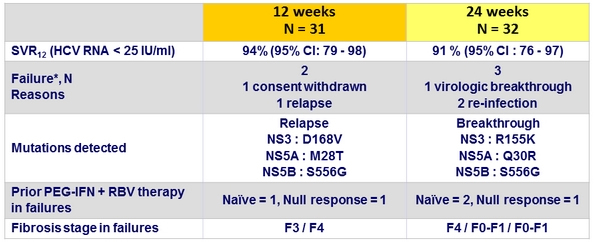
* All 5 = genotype 1a
- No HIV RNA failure (3 blips in W12-group and 5 blips in W24-group)
- Decrease in absolute but not relative CD4 cell counts
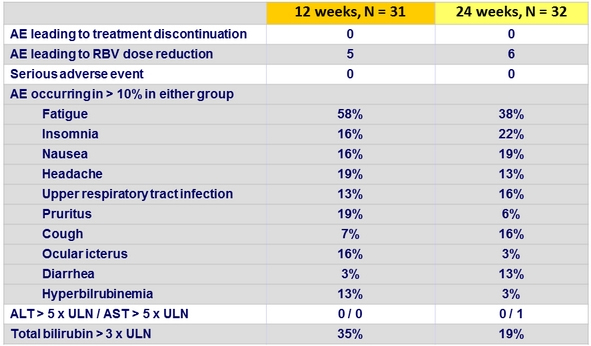
Adverse events, Part 1a, n (%)
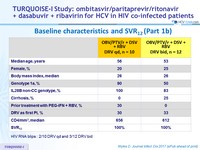
Baseline characteristics and SVR12 (Part 1b)
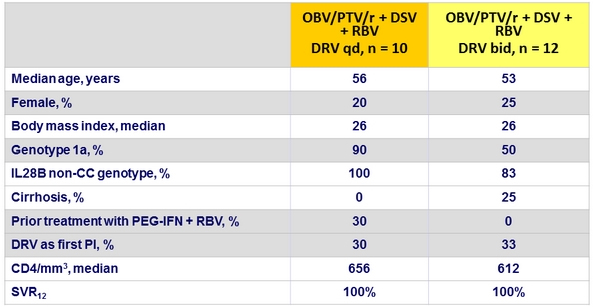
HIV RNA blips : 2/10 DRV qd and 3/12 DRV bid
Pharmacokinetic parameters of DRV qd and bid (Part 1b)
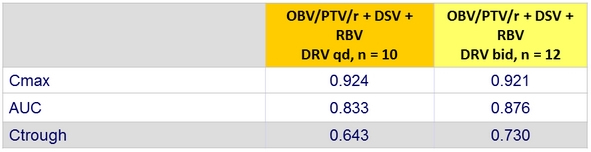
Values are the least square mean ratios (90% confidence intervals) for DRV pharmacokinetic parameters with and without OBV/PTV/r + DSV (DRV + OBV/PTV/r + DSV vs DRV alone)
Adverse events, Part 1b, n
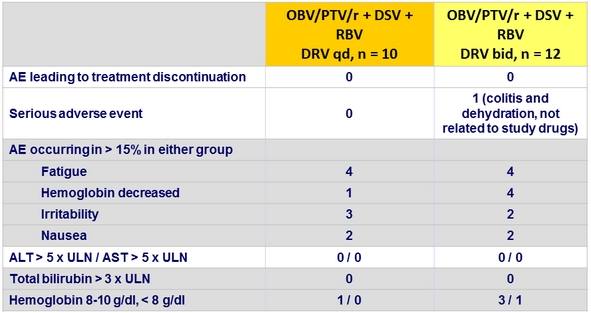
RBV dose reduction, n = 9 (decrease hemoglobin levels, n = 4 ; anemia, n = 3 ; fatigue, n = 2)
Summary
- In Part 1a of this open-label, randomised uncontrolled study, treatment with the all-oral, IFN-free 3D plus ribavirin regimen resulted in high SVR12 rates among patients co-infected with HCV genotype 1 and HIV-1 whether treated for 12 or 24 weeks
- No discontinuation for AE
- The 2 failures (1 virologic breakthrough and 1 relapse) were observed in patients with genotype 1a and F4 fibrosis
- Limitations
- Small sample size
- ARV therapy limited to ATV/r- or RAL-containing regimens
- Role of RBV not addressed
- In Part 1b, HCV genotype 1 HIV-1 coinfected patients on stable DRV-containing ART achieved 100% SVR12 while maintaining plasma HIV-1 RNA suppression.
- Despite DRV Ctrough decrease, episodes of intermittent HIV-1 viremia were infrequent