TURQUOISE-II Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV with cirrhosis
ABT-450/r–Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis
Poordad F, NEJM 2014;370:1973-82
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
1
1a
1b
Naive
IFN-Experienced
Yes
Design
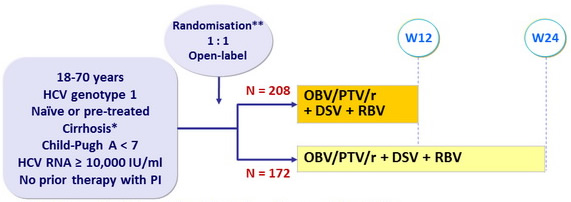
* Liver biopsy with Metavir > 3 or Ishak > 4, or Fibroscan kPa > 14.6
** Randomisation stratified on prior Peg-IFN + RBV therapy ;
Naïve patients, stratified on genotype (1a vs 1b) and IL-28B (CC vs non-CC)
Experienced patients stratified on genotype subtype, prior response (null, partial, relapse)
Treatment regimens
- Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg)
Primary efficacy endpoint
- Sustained virologic response (HCV RNA < 25 IU/ml) 12 weeks after end of treatment, with two-sided 97.5% CI
- Non-inferiority of SVR12 assessed vs estimated rate of SVR24 with a telaprevir-based regimen (47%; 95% CI : 41 to 54). A noninferiority margin of 10.5 % established 43% as the noninferiority threshold; the superiority threshold was 54%
- Analyses by mITT
HCV RNA
- COBAS TaqMan real-time RT–PCR assay, v 2.0 (Roche)
Virologic failure
- 2 consecutive HCV RNA > 1 log10 IU/ml above the nadir at any time during treatment,
- HCV RNA ≥ 25 IU/ml at all assessments during treatment among patients who received at least 6 weeks of treatment,
- confirmed HCV RNA ≥ 25 IU/ml after a level < 25 IU/ml during treatment
Virologic relapse
- confirmed HCV RNA ≥ 25 IU/ml between the end of treatment and 12 weeks after the last dose of study drug among patients who completed treatment and had an HCV RNA < 25 IU/ml at the final visit during the treatment period
Exploratory analysis
- stepwise logistic-regression model to assess the association between the rate of SVR12 and continuous and categorical subgroup variables
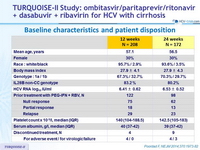
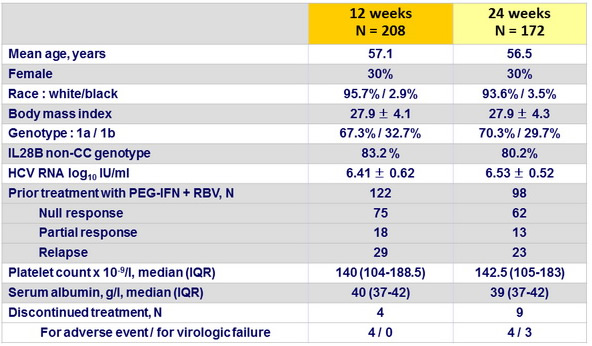
Baseline characteristics and patient disposition
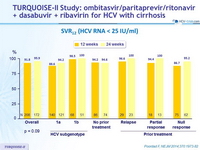
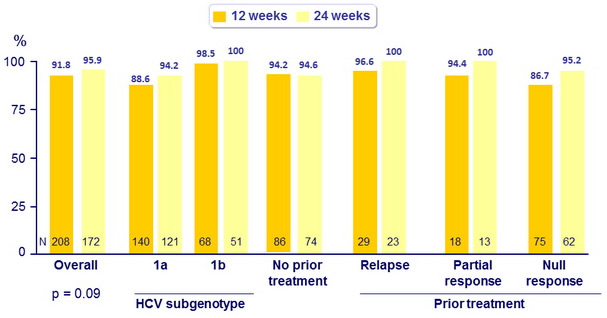
SVR12 (HCV RNA < 25 IU/ml)
Outcomes for patients without SVR12
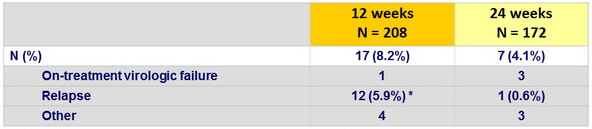
* 7/12 had genotype 1a and prior null response to PEG-IFN + RBV
Resistance testing (population sequencing) of the 17 virologic failures (on-treatment or relapse)
- No resistance, N = 2
- Resistance, N = 15
- D168V (NS3) and Q30R (NS5A) most frequent in genotype 1a
- D168V (NS3), Y93H (NS5A) and C316Y + M414I (NS5B) in the single genotype 1b case
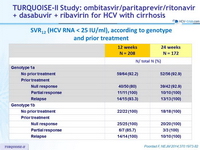
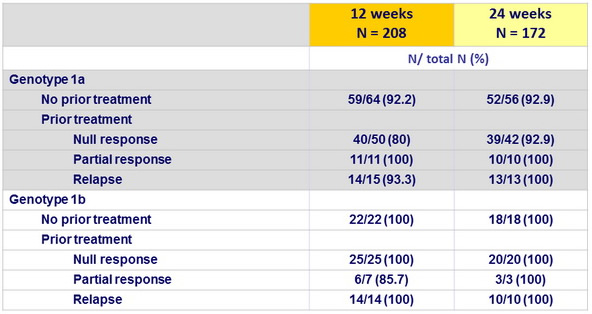
SVR12 (HCV RNA < 25 IU/ml), according to genotype and prior treatment
Multivariate Analysis of Association of Variables with a SVR12
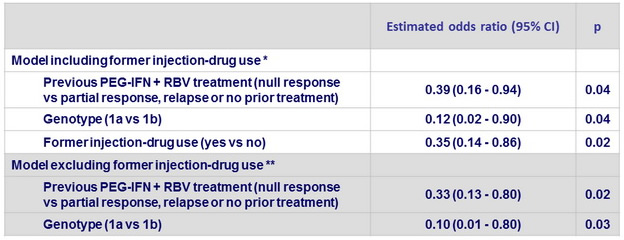
* Also adjusted for baseline Child–Pugh score and ethnic group; ** also adjusted for geographic region and treatment duration
Continuous variables : age, BMI, platelet, albumin, alpha-foetoprotein, HCV RNA level
Categorical variables : treatment duration, genotype, IL28B genotype, previous PEG-IFN + RBV, sex, race, ethnicity, geographic region, Child–Pugh score, diabetes, history of depression or bipolar disorder , former injection-drug use
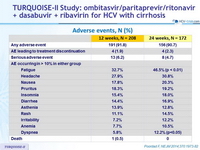
Adverse events, N (%)
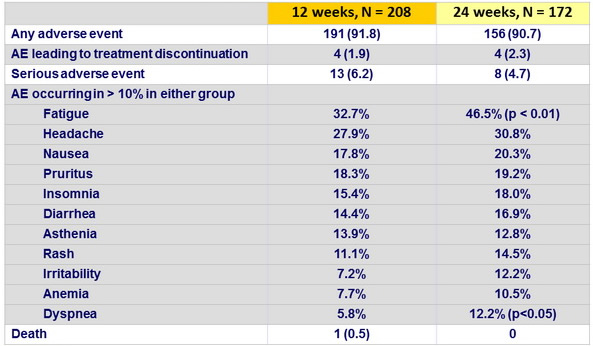
AE leading to discontinuation

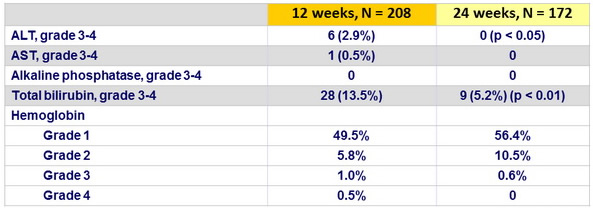
Laboratory abnormalities, N (%)
Summary
- In both treatment groups of 3D + RBV, the primary efficacy end points met the prespecified criteria for noninferiority and superiority to the historical rate with telaprevir plus PEG-IFN + RBV among patients with HCV genotype 1 infection and cirrhosis
- 12 or 24 weeks of treatment with coformulated ombitasvir/paritaprevir/ritonavir and dasabuvir, administered with RBV, resulted in high rates of SVR12 (91.8% and 95.9%, respectively)
- Patients with cirrhosis and a prior null response had SVR12 of 86.7% and 95.2% in the 12-W and 24-W groups, respectively.
- Patients with genotype 1a and a prior null response had a SVR12 of 80% with 12 weeks of treatment and 92.9% with 24 weeks of treatment.
- All other subgroups with genotype 1a, including previously naïve patients, those with a prior partial response, with a prior relapse, had rates of SVR12 of 92 to 100% with 12 weeks or 24 weeks of treatment
- Patients with genotype 1b and a prior null response had a SVR12 of 100% with either treatment duration
- Drug discontinuations due to adverse events were infrequent