ALLY-2 Study: DCV + SOF for HCV in HIV co-infection
Daclatasvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1
Wyles DL. N Engl J Med. 2015 Aug 20;373(8):714-25.
Anti-HCV
Daclatasvir
Sofosbuvir
Daclatasvir
Sofosbuvir
Genotype
1a
1a
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Special population
HIV co-infection
HIV co-infection
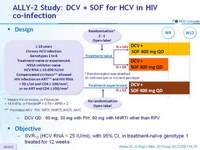
Design
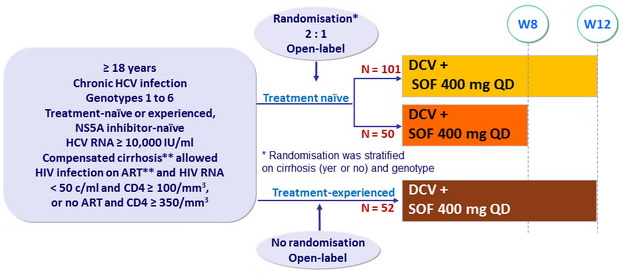
* Metavir F4 on biopsy, or Fibroscan > 14.6 kPa , or Fibrotest ® > 0.74 + APRI > 2
- DCV QD : 60 mg, 30 mg with PI/r, 90 mg with NNRTI other than RPV
Objective
- SVR12 (HCV RNA < 25 IU/ml), with 95% CI, in treatment-naïve genotype 1 treated for 12 weeks
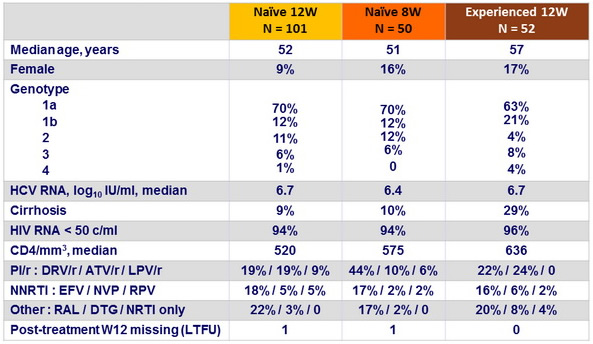
Baseline characteristics and patient disposition
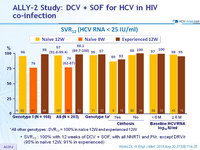
SVR12 (HCV RNA < 25 IU/ml)
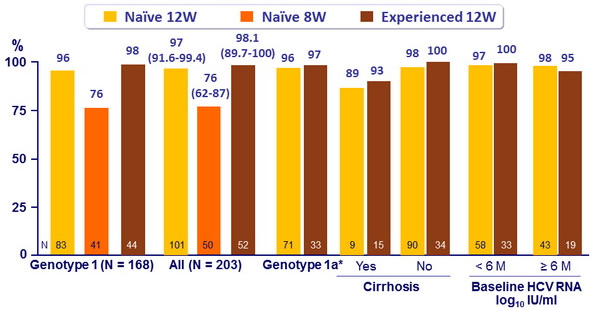
* All other genotypes : SVR12 = 100% in naïve 12W and experienced 12W
- SVR12 : 100% with 12 weeks of DCV + SOF, with all NNRTI and PI/r, except DRV/r (95% in naïve 12W, 91% in experienced)
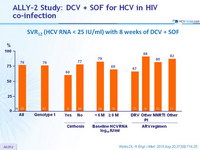
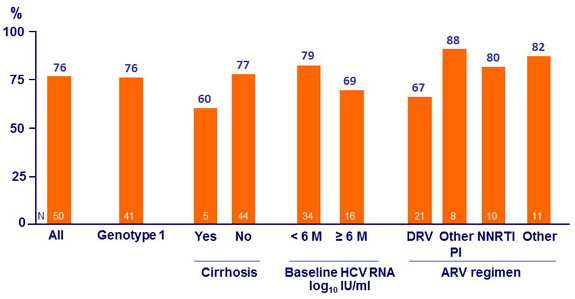
SVR12 (HCV RNA < 25 IU/ml) with 8 weeks of DCV + SOF
Resistance data
- NS5B RAVs at baseline in 1/39 tested patients (C316H + V321I)
- 17% (33/198) of baseline sequences had NS5A polymorphisms at positions 28, 30, 31 or 93
Similar SVR12 rates in patients with or without baseline NS5A RAVs

- 12 patients with relapse
- 10 in 8-week arm, 9/10 with concomitant DRV/r
- 2 / 10 relapses had baseline NS5A RAVs, 1 of which had also NS5B-V321I RAV
- 1 /10 relapses in 8-week arm had an emergent NS5A RAV at time of failure (Q30E)
- No other RAVs emergence at failure in the remaining 6 cases
- 2 in 12-week arm
- 1 had a NS5A RAV at baseline and failure (Y93N), and NS5B-L159F variant at failure
- 1 had emergence of NS5A-Q30R at failure
Adverse events and Grade 3-4 laboratory abnormalities, N (%)
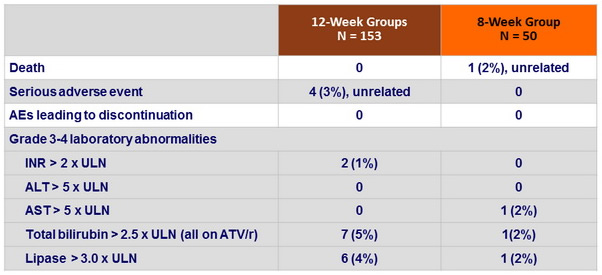
- 2 HIV virologic failure, 1 unconfirmed with treatment discontinuation,
- 1 with HIV resuppressed with no ART adjustment
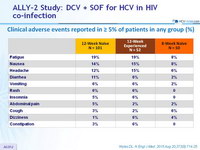
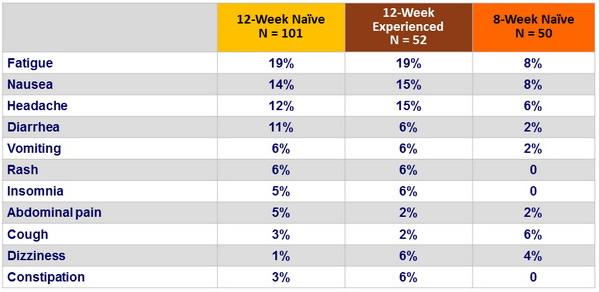
Clinical adverse events reported in = 5% of patients in any group (%)
Summary
- After 12 weeks of DCV + SOF in HIV/HCV coinfected patients, the overall SVR12 was 97%
- 97% in GT 1 ; 100% in GT 2, 3, and 4
- 97% in treatment-naive and 98% in treatment-experienced patients
- No significant effect of race, baseline HCV RNA levels, cirrhosis or ARV regimen
- In patients treated for 8 weeks with DCV + SOF, SVR12 was 76%
- Increased relapse in coinfected patients with shorter therapy, higher baseline HCV RNA (= 2M IU /ml) , and DRV/r-based cART with DCV 30 mg QD
- No compromise of HIV suppression and no modification of on-treatment ARV regimens due to DCV + SOF
- DCV + SOF was safe and well tolerated, offers a predictable drug-drug interaction profile with flexibility to dose adjust, and is compatible with a wide range of antiretrovirals