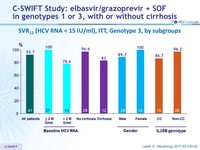
C-SWIFT Study: elbasvir/grazoprevir + SOF in genotypes 1 or 3, with or without cirrhosis
Lawitz E. Hepatology 2017;65:439-50
Anti-HCV
Grazoprevir
Elbasvir
Sofosbuvir
Grazoprevir
Elbasvir
Sofosbuvir
Genotype
1a
1b
3
1a
1b
3
Treatment history
Naive
SOF-experienced
Naive
SOF-experienced
Cirrhosis
Yes
No
Yes
No
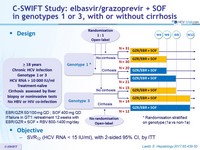
Design
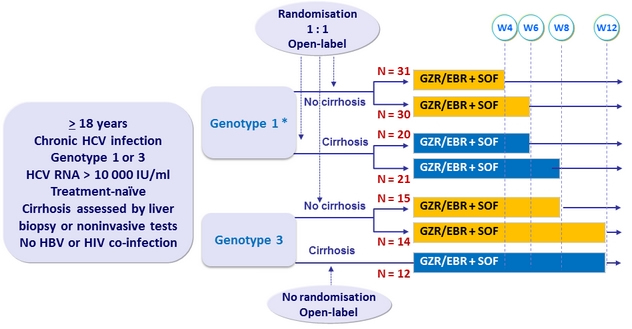
* Randomisation stratified
on genotype (1a vs non-1a)
EBR/GZR 50/100 mg QD ; SOF 400 mg QD
If failure in GT1: retreatment 12 weeks with EBR/GZR + SOF + RBV 800-1400 mg/day
Objective
- SVR12 (HCV RNA < 15 IU /ml) , with 2-sided 95% CI, by ITT
Baseline characteristics, and disposition
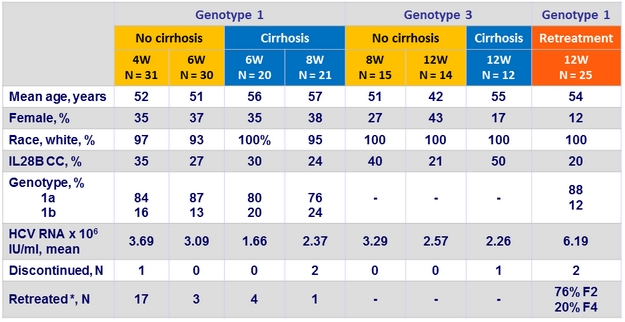
* Days from virologic failure to retreatment = 214 (range: 182-260)
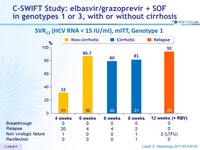
SVR12 (HCV RNA < 15 IU/ml), mITT , Genotype 1
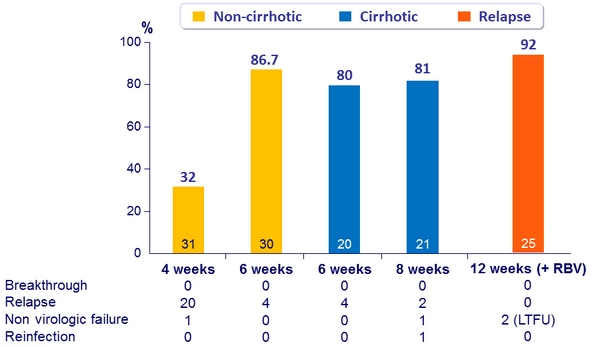
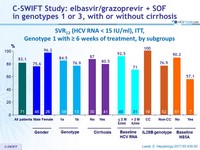
SVR12 (HCV RNA < 15 IU/ml), ITT, Genotype 1 with = 6 weeks of treatment, by subg roups
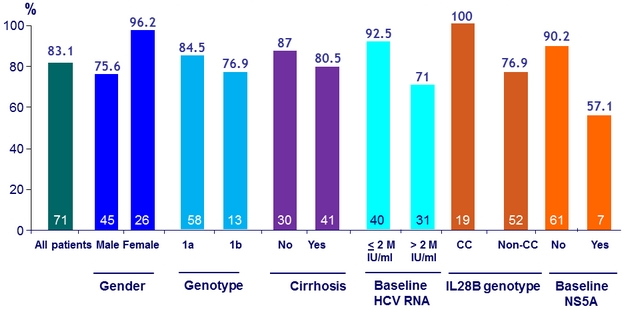
Impact of RAVs on SVR12 in genotype 1
- NS3
- Prevalence at baseline = 66%
- No impact on SVR12 : 76% if no baseline NS3 RAVs vs 69% if present
- NS5A
- Prevalence at baseline in the 6- and 8-week groups = 10%
- SVR12 : 90% if no baseline RAVs vs 57% if present
- Retreatment group (next generation sequencing analysis, 1% sensitivity threshold) •
- Baseline NS5A RAVs = 14/23 (61%)
- Baseline NS3 RAVs = 17/23 (74%)
- NS3 + NS5A RAVs = 11/23 (48%)
- SVR12 = 23/23 (2 lost to follow-up)
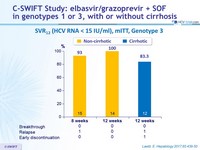
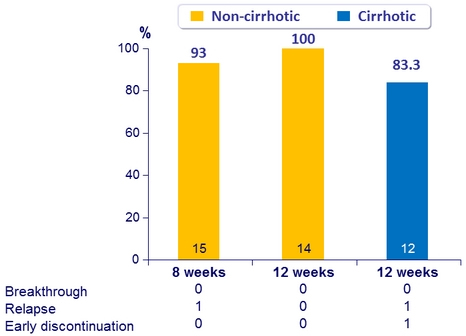
SVR12 (HCV RNA < 15 IU/ml), mITT, Genotype 3
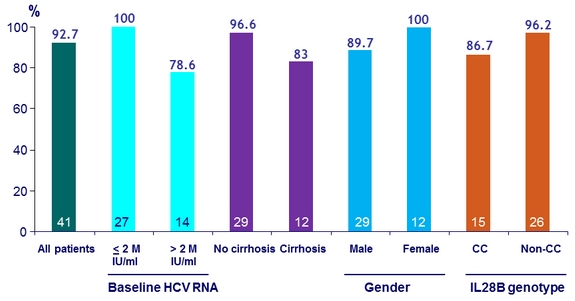
SVR12 (HCV RNA < 15 IU /ml), ITT, Genotype 3, by subgroups
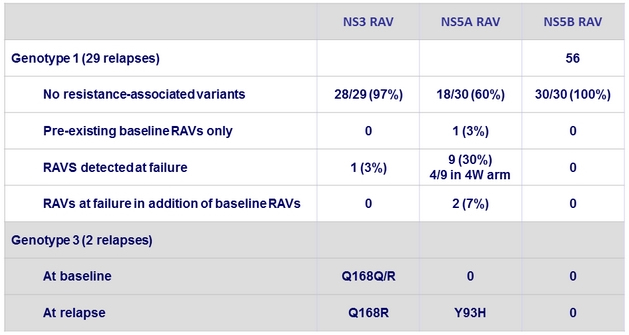
Resistance analysis at failure
Adverse events, N (%)
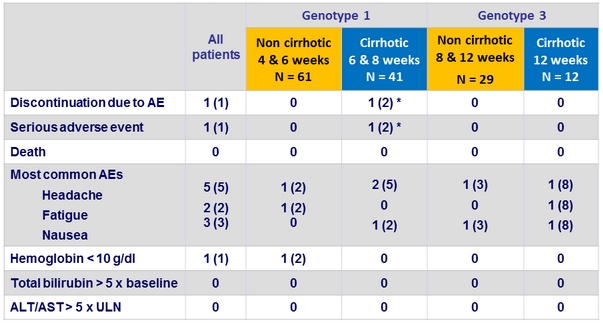
* Pneumonia on day 40 of treatment, which led to discontinuation of therapy
Summary
- Elbasvir/grazoprevir + sofosbuvir was able to shorten treatment duration to 8 weeks or less among cirrhotic and non-cirrhotic HCV genotype 1 infected patients
- Genotype 3 patients achieved high SVR12 rates with 8-12 weeks of therapy, including patients with cirrhosis
- All virologic failures were due to relapse
- Patients relapsed most commonly with either wild-type virus or with RAVs already present at baseline
- GZR/EBR + SOF was generally safe and well tolerated
- Retreatment of the patients who failed short-duration therapy was successfully achieved through extended treatment duration (12 weeks) and the addition of ribavirin