Japanese 2D Study: OBV/PTV/r in treatment-experienced Japanese patients
Randomized Trial of Interferon- and Ribavirin-Free Ombitasvir/Paritaprevir/Ritonavir in Treatment-Experienced Hepatitis C Virus–Infected Patients
Chayama K. Hepatology 2015;61:1523-32
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Ombitasvir
Paritaprevir/ritonavir
Genotype
1b
2
1b
2
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
No
No
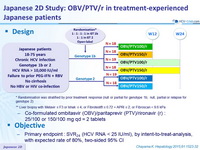
Design
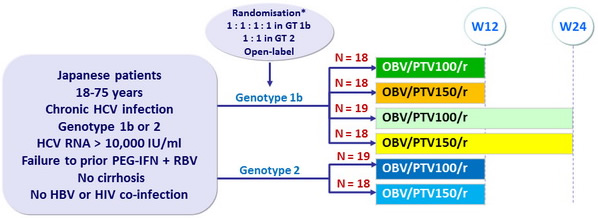
* Randomisation was stratified by prior treatment response (null or partial for genotype 1b; null, partial or relapse for genotype 2)
** Liver biopsy with Metavir = F3 or Ishak = 4, or Fibrotest® = 0.72 + APRI = 2, or Fibroscan < 9.6 kPa
- Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/100 or 150/100 mg qd = 2 tablets
Objective
- Primary endpoint : SVR 24 (HCV RNA < 25 IU /ml) , by intent-to-treat-analysis, with expected rate of 80%, two-sided 95% CI
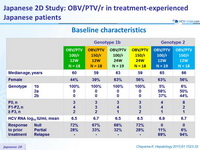
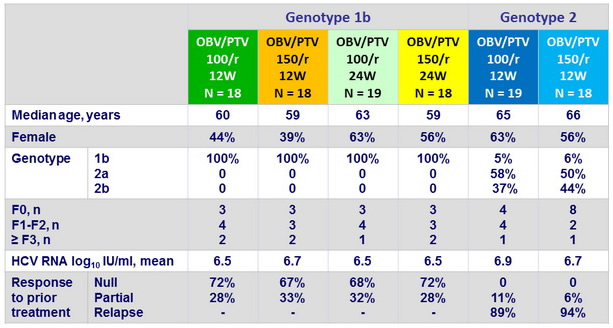
Baseline characteristics
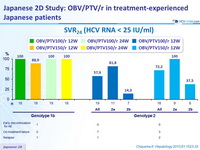
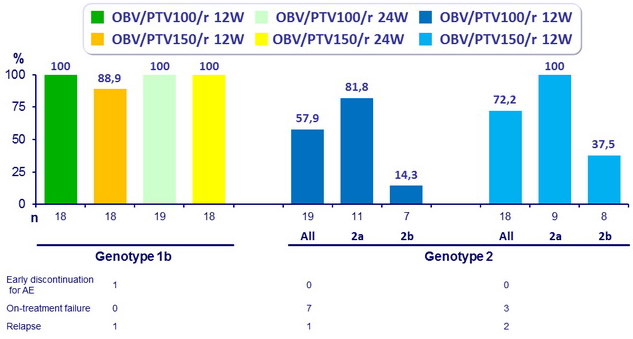
SVR 24 (HCV RNA < 25 IU /ml)
Virologic failure
- Genotype 1b
- The patient who relapsed had resistance-associated variants in NS3 (D168V) and NS5A (Y93H) at the time of virologic failure
- The NS5A RAV, Y93H, was present at baseline in 4 patients with genotype 1b ; all achieved SVR 24
- Genotype 2
- All patients with virologic failure had resistance-associated variants in NS3 and/or NS5A at the time of virologic failure, most commonly D168V or D168Y in NS3 and L28F in NS5A
- The presence of RAVs in NS3 and NS5A at baseline did not impact response
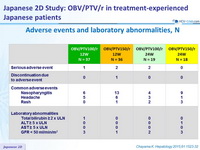
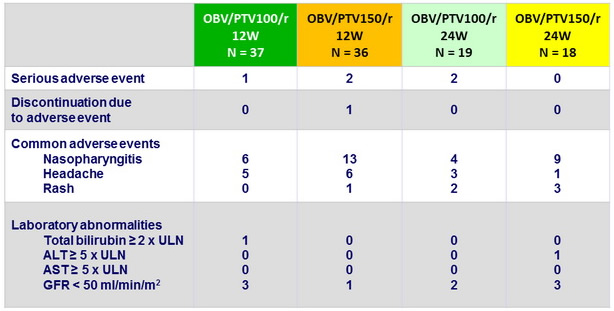
Adverse events and laboratory abnormalities, N
Summary
- In this randomised phase II study of an IFN- and RBV-free regimen of ombitasvir / paritaprevir / ritonavir in PEG-IFN + RBV treatment–experienced Japanese patients
- High SVR 24 rates were observed in patients with HCV subtype 1b infection, regardless of paritaprevir dose or treatment duration.
- Among patients with genotype 2 infection, SVR rates were higher in those receiving paritaprevir 150 mg and those with subtype 2a infection
- These differences in response rates for genotype 2 may be related to NS3/4A or NS5A polymorphisms between subtypes 2a and 2b
- Together, these results show promising antiviral activity for this all-oral 2 DAA combination regimen
- Safety and tolerability was good
- Limitations
- Exclusion of patients with cirrhosis
- Relatively small number of patients in each treatment arm