M14-103 Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + RBV for genotype 1 on opioid replacement therapy
Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine
Lalezari J. J Hepatology 2015;63:364-9
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1
1a
1
1a
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Special population
Opioid replacement
Opioid replacement
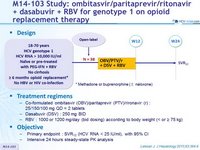
Design
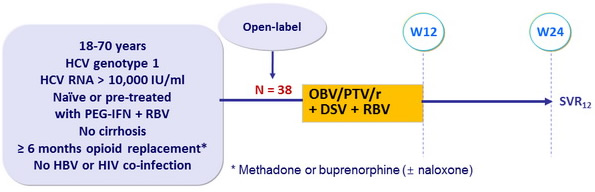
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r) : 25/150/100 mg QD = 2 tablets
- Dasabuvir (DSV) : 250 mg BID
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg )
Objective
- Primary endpoint : SVR12 (HCV RNA < 25 IU/ml), with 95% CI
- Intensive 24 hours steady-state PK analysis
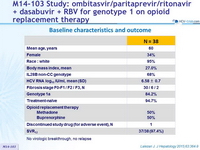
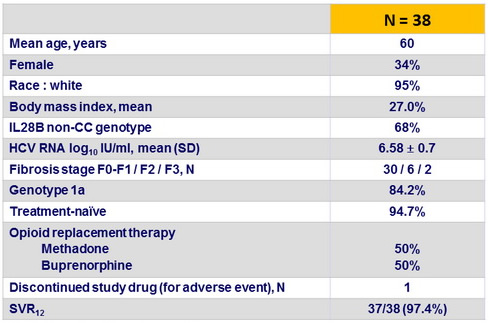
Baseline characteristics and outcome
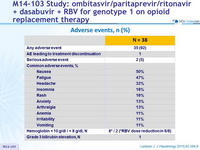
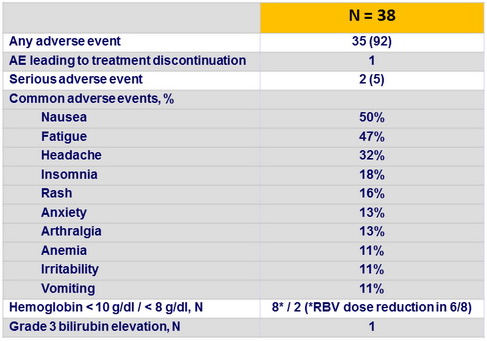
Adverse events, N (%)
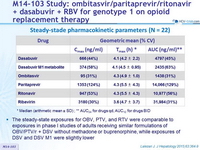
Steady- stade pharmacokinetic parameters (N = 22)
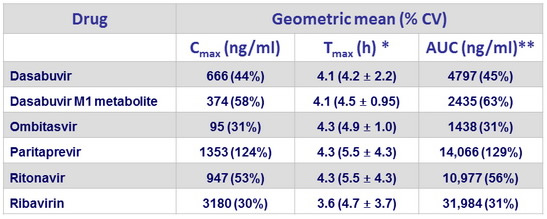
* Median (arithmetic mean ± SD) ; ** AUC 24 for drugs qd , AUC 12 for drugs BID
- The steady-state exposures for OBV, PTV, and RTV were comparable to exposures in phase I studies of adults receiving similar formulations of OBV/PTV/r + DSV without methadone or buprenorphine, while exposures of DSV and DSV M1 were slightly lower
Summary
- The 3D + RBV regimen achieved an SVR12 rate of 97.4% among these 38 genotype 1- infected patients receiving opioid replacement therapy
- No viral breakthroughs or relapses were observed
- The regimen was well tolerated, with low rate of discontinuation
- Adverse events were generally mild
- Drug-drug interactions did not impact HCV treatment or opioid maintenance
- No patient required a change in the dosage of methadone or buprenorphine during study treatment
- 12 weeks all -oral regimen of OBV/PTV/r + DSV + RBV is well-tolerated and may be an attractive treatment option for genotype 1 infected patients receiving opioid replacement therapy