PHOTON-2 Study: SOF + RBV in HCV-HIV co-infection
Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study
Molina JM. Lancet 2015;385:1098-1106
Anti-HCV
Sofosbuvir
Ribavirin
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
2
3
4
1
1a
1b
2
3
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Special population
HIV co-infection
HIV co-infection
Design
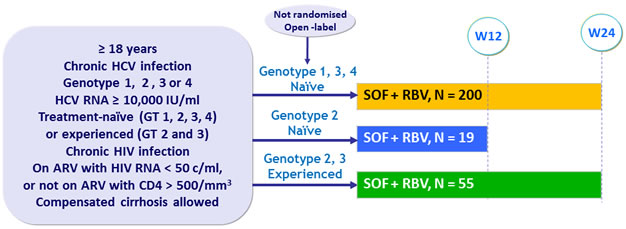
- SOF : 400 mg qd
- RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if = 75 kg
Objective
- SVR12 with 2-sided 95% CI, descriptive analysis
- Multivariate analyses of predictors of SVR12
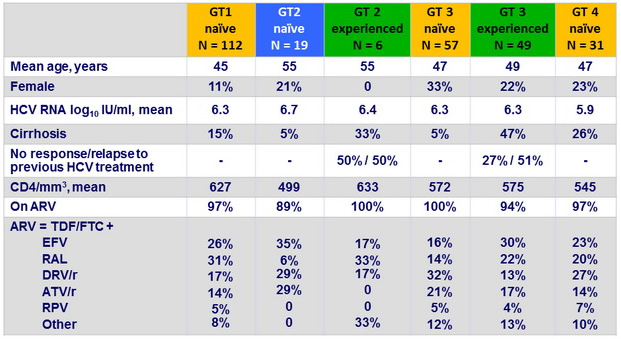
Baseline characteristics
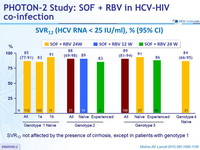
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
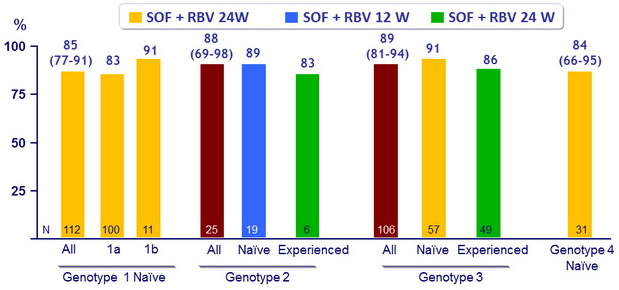
- SVR12 not affected by the presence of cirrhosis, except in patients with genotype 1
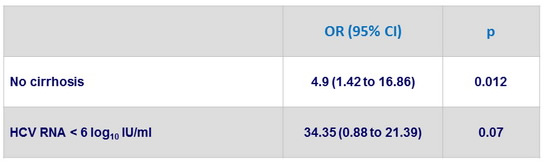
Multivariate analysis of factors associated with SVR12 in genotype 1
Virologic failure
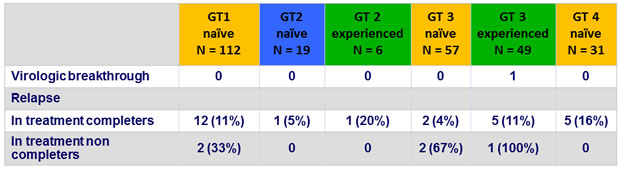
Deep sequencing of NS5B in 31 failures
- No emergence of S282T variant
- Low-levels of emergent variants, N = 2
- 1 virologic breakthrough, genotype 3a, L159F variant
- 1 with slow response, genotype 3a, L159N + S282N + V321A variants
- 2 patients (genotype 1, genotype 3a) with L159F at relapse
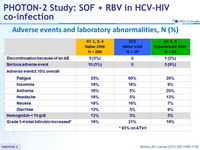
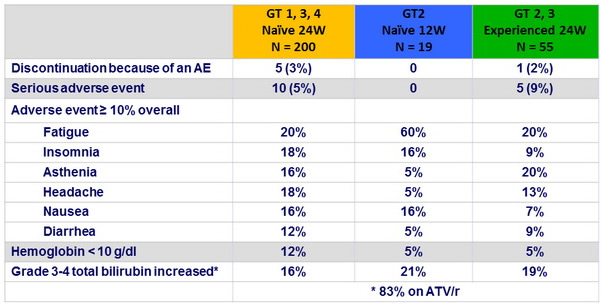
Adverse events and laboratory abnormalities, N (%)
Summary
- Patients co-infected with HIV and HCV genotypes 1- 4 achieved high SVR12 with an interferon-free regimen of SOF plus RBV
- For treatment-naïve genotypes 1, 3, 4, SVR12 was 84-91% with 24 weeks of SOF + RBV
- Higher SVR12 in genotype 1b (91%) than in genotype 1a (84%)
- Presence of cirrhosis was a negative predictor of SVR12 only in genotype 1 patients
- For treatment-naïve genotype 2, SVR12 was 89% with 12 weeks of SOF + RBV
- For treatment-experienced genotypes 2 and 3, SVR12 was 83-86% with 24 weeks of SOF + RBV
- For treatment-naïve genotypes 1, 3, 4, SVR12 was 84-91% with 24 weeks of SOF + RBV
- Compared to studies in HCV mono-infection, HIV co-infection does not have a negative effect on response to SOF-based therapy
- Unclear clinical relevance of NS5B variants emerging at failure