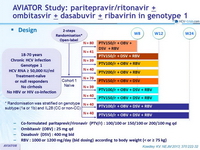
AVIATOR Study: paritepravir /ritonavir ± ombitasvir ± dasabuvir ± ribavirin in genotype 1
Phase 2b Trial of Interferon-free Therapy for Hepatitis C Virus Genotype 1
Kowdley KV. NEJM 2013; 370:222-32
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
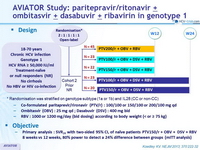
Design
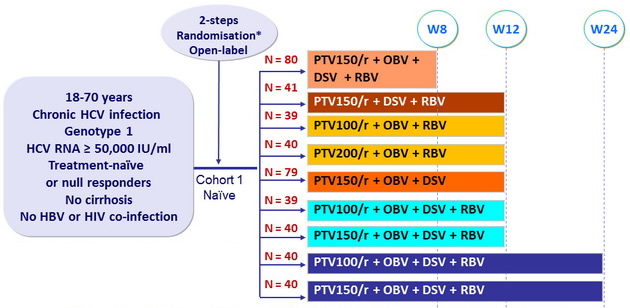
*Randomisation was stratified on genotype subtype (1a or 1b) and IL28 (CC or non-CC)
- Co-formulated paritaprevir / rironavir (PTV/r) : 100/100 or 150/100 or 200/100 mg qd
- Ombitasvir (OBV) : 25 mg qd
- Dasabuvir (DSV) : 400 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Design
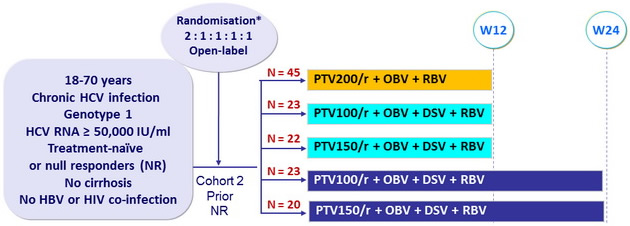
*Randomisation was stratified on genotype subtype (1a or 1b) and IL28 (CC or non-CC)
- Co-formulated paritaprevir / rironavir (PTV/r) : 100/100 or 150/100 or 200/100 mg qd
- Ombitasvir (OBV) : 25 mg qd ; Dasabuvir (DSV) : 400 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Objective
- Primary analysis : SVR24 with two-sided 95% CI, of naïve patients PTV150/r + OBV + DSV + RBV 8 weeks vs 12 weeks, 80% power to detect a 24% difference between groups ( mITT analysis)
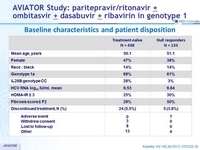
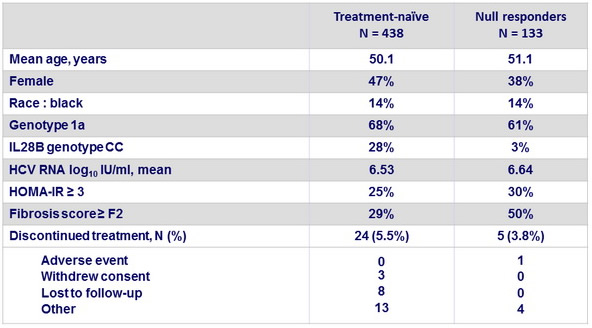
Baseline characteristics and patient disposition
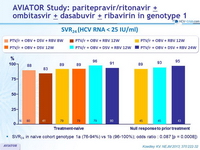
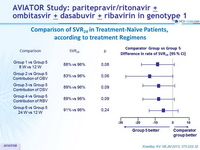
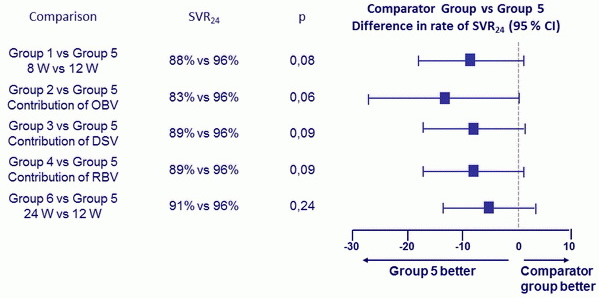
SVR24 (HCV RNA < 25 IU/ml)

- SVR24 in naïve cohort genotype 1a (76-94%) vs 1b (96-100%); odds ratio : 0.087 [p = 0.0008])
Comparison of SVR24 in Treatment -Naïve Patients, according to treatment Regimens
Resistance emergence
- No variants at relapse in 7/10 patients in the 8-week groups
- In the 12-week and 24-week groups, 31/32 samples at breakthrough or relapse showed emergence of resistant variants.
Most common :- NS3 : position 168
- NS5A : positions 28 and 30
- NS5B : position 556
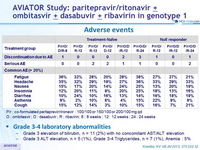
Adverse events
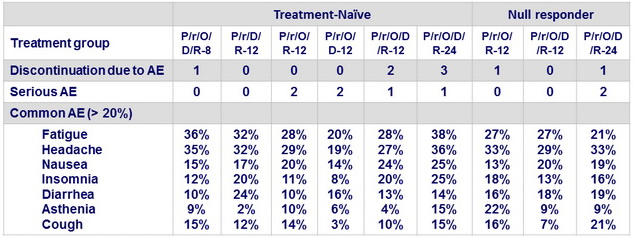
P/r : co-formulated paritaprevir / rironavir : 100/100 or 150/100 or 200/100 mg qd
O : ombitasvir ; D : dasabuvir ; R : ribavirin ; 8 : 8 weeks ; 12 : 12 weeks ; 24 : 24 weeks
Grade 3-4 laboratory abnormalities
- Grade 3 elevation of bilirubin , n = 11 (2%) with no concomitant AST/ALT elevation
- Grade 3 ALT elevation, n = 5 (1%), Grade 3-4 Triglycerides, n = 7 (1%), Anemia : 5%
Summary
- In this phase IIb study, all-oral regimens of antiviral agents and RBV were effective both in non-cirrhotic patients with HCV genotype 1 infection who had not received therapy previously and in those who had not had a response to prior therapy
- Rates of SVR24 ranged from 83% to 100%
- Among previously untreated patients, the rate of treatment failure was lower among those receiving the triple combination of paritaprevir /ritonavir + ombitasvir + dasabuvir + RBV for 12 weeks than among those who received the same regimen for 8 weeks and among those who received fewer agents; extending the treatment to 24 weeks offered no further benefit
- Higher number of relapses in 3D + RBV 8 weeks vs 12 weeks
- Paritaprevir /ritonavir + ombitasvir + dasabuvir + RBV for 12 weeks was associated with SVR24 of 93% in null responders to prior therapy
- For genotype 1b, all regimens led to SVR24 of 94%-100% with only 1/24 relapse in the 8-week group