PROMISE Study: SMV + PEG-IFN + RBV for genotype 1 and relapse to prior IFN therapy
Simeprevir With Peginterferon and Ribavirin Leads to High Rates of SVR in Patients With HCV Genotype 1 Who Relapsed After Previous Therapy: A Phase 3 Trial
Forns X. Gastroenterology 2014;146:1669-79
Anti-HCV
Simeprevir
PEG-IFNα 2a
Ribavirin
Simeprevir
PEG-IFNα 2a
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
IFN-Experienced
IFN-Experienced
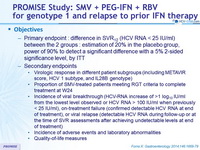
Design
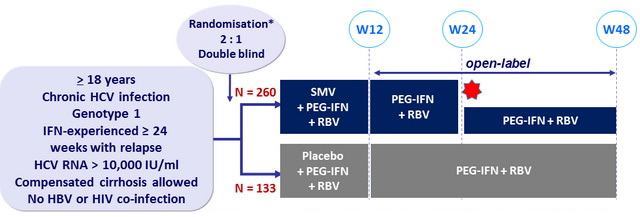
*Randomisation was stratified on genotype (1a or 1b or other) and IL28B genotype (CC, CT or TT)
- SMV 150 mg : 1 pill QD ; PEG-IFNα -2a 180 m g SC once weekly
- RBV : 1000 or 1200 mg/day (BID dosing) according to body weight (< or = 75 kg)
Response-guided therapy :
- in SMV group, patients with HCV RNA < 25 IU/ml at W4 and < 15 IU/ml at W12 stopped treatment at W24, otherwise they continued until W48
Virological stopping rules :
- SMV or placebo discontinued if HCV RNA > 1000 IU/ml at W4, with continuation of PEG-IFN + RBV. PEG-IFN + RBV discontinued if RNA reduction < 2 log 10 IU/ml at W12, or if HCV RNA confirmed = 25 IU/ml at W24 or W36
Objectives
- Primary endpoint : difference in SVR12 (HCV RNA < 25 IU/ml) between the 2 groups : estimation of 20% in the placebo group, power of 90% to detect a significant difference with a 5% 2-sided significance level, by ITT
- Secondary endpoints
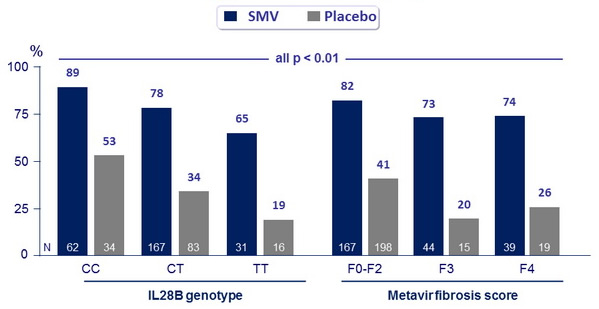
- Virologic response in different patient subgroups (including METAVIR score, HCV 1 subtype , and IL28B genotype )
- Proportion of SMV-treated patients meeting RGT criteria to complete treatment at W24
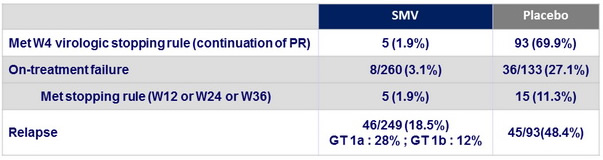
- Incidence of viral breakthrough (HCV-RNA increase of > 1 log 10 IU/ ml from the lowest level observed or HCV RNA > 100 IU/ ml when previously < 25 IU/ ml) , on-treatment failure ( confirmed detectable HCV RNA at end of treatment) , or viral relapse (detectable HCV RNA during follow-up or at the time of SVR assessments after achieving undetectable levels at end of treatment)
- Incidence of adverse events and laboratory abnormalities
- Quality -of-life measures
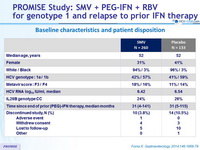
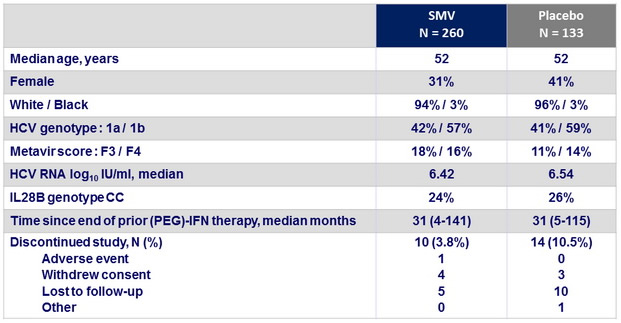
Baseline characteristics and patient disposition
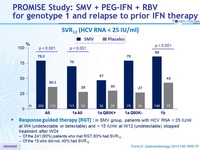
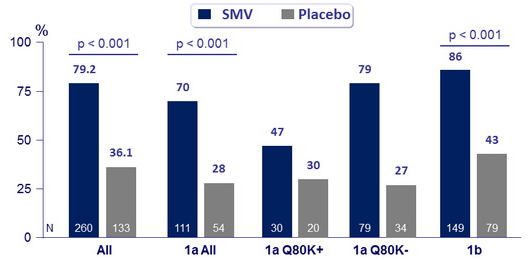
SVR12 (HCV RNA < 25 IU/ml)
- Response guided therapy (RGT) : in SMV group, patients with HCV RNA < 25 IU /ml at W 4 (undetectable or detectable) and < 15 IU /ml at W 12 (undetectable) stopped treatment after W24
- Of the 241 (93%) patients who met RGT, 83% had SVR12
- Of the 15 who did not, 40% had SVR12
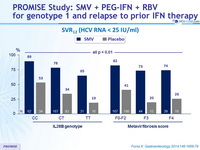
SVR12 (HCV RNA < 25 IU/ml)
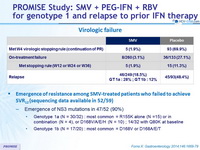
Virologic failure
- Emergence of resistance among SMV-treated patients who failed to achieve SVR12 (sequencing data available in 52/59)
- Emergence of NS3 mutations in 47/52 (90%)
- Genotype 1a (N = 30/32) : most common = R155K alone (N =15) or in combination (N = 4), or D168V/A/E/H (N = 10) ; 14/32 with Q80K at baseline
- Genotype 1b (N = 17/20) : most common = D168V or D168A/E/T
- Emergence of NS3 mutations in 47/52 (90%)
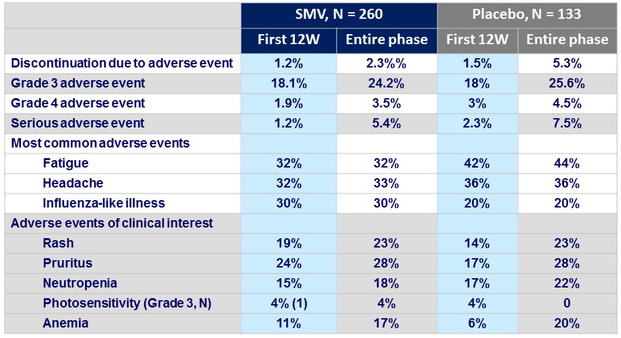
Adverse events
Summary
- Oral, once-daily SMV150 mg for 12 weeks in combination with PEG-IFN + RBV followed by 12 –36 weeks of PEG-IFN + RBV led to a significant improvement in SVR12 compared with that seen in the placebo group, in patients with chronic HCV genotype 1 infection who had relapsed after previous IFN-based therapy
- Overall , 79.2 % of SMV- treated patients achieved SVR12 compared with 36.1% of those who received PEG-IFN + RBV alone
- This superiority of SMV was seen across the different baseline characteristics (Gender, HCV RNA, Metavir score, IL28B genotype, HCV 1 subtype)
- Almost all SMV- treated patients (92.7%) met RGT criteria and were eligible to stop PEG-IFN + RBV at W 24 . The SVR12 rate in these patients was 83%
- The SVR12 rate with SMV was lower in HCV genotype 1a patients who had the Q80K polymorphism at baseline
- Relapse rate was higher in genotype 1a
- Most patients treated with SMV who did not achieve SVR12 had emergent mutations at the time of failure
- The safety and tolerability profile of SMV + PEG-IFN + RBV was generally similar to that of PEG-IFN + RBV alone, with no additional treatment-related adverse events. Discontinuation for adverse event was rare in both groups