TURQUOISE-III Study: ombitasvir/paritaprevir/ritonavir + dasabuvir for genotype 1b with cirrhosis
Safety and Efficacy of 12-Week Ribavirin-Free Treatment for Patients with HCV Genotype 1B and Cirrhosis
Feld JJ. J Hepatol 2016; 64:301-7
Anti-HCV
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Ombitasvir
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1b
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
Yes
Design
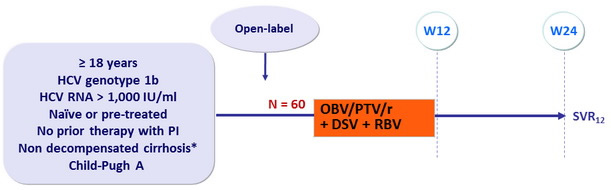
* Liver biopsy with Metavir > 3 or Ishak > 4, or Fibroscan > 14.6 kPa
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r) : 25/150/100 mg QD = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
Objective
- SVR12 (HCV RNA < 25 IU/ml)
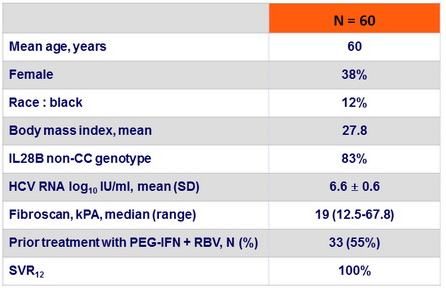
Baseline characteristics and outcome
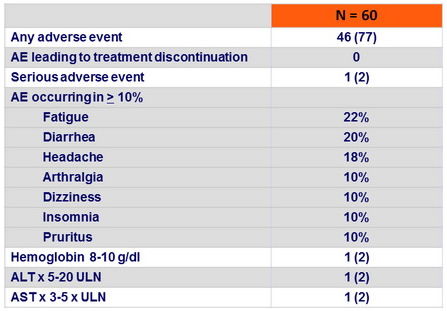
Adverse events, N (%)
Summary
- OBV/PTV/r FDC + DSV, without the use of RBV, given for 12 weeks achieved SVR12 of 100% in genotype 1b infected patients with compensated cirrhosis, including treatment-experienced patients
- Treatment was very well tolerated, with a low rate of serious adverse events, no premature discontinuations, and infrequent laboratory abnormalities that were not clinically relevant




