BOSON Study: SOF + RBV ± PEG-IFN for genotypes 2 and 3
Sofosbuvir Plus Peg-IFN/RBV for 12 Weeks vs Sofosbuvir/RBV for 16 or 24 Weeks in Genotype 3 HCV-Infected Patients and Treatment-Experienced Cirrhotic Patients With Genotype 2 HCV: The BOSON Study
Foster GR. Gastroenterology. 2015 Nov;149(6):1462-70
Anti-HCV
Sofosbuvir
Ribavirin
PEG-IFNα 2a
Sofosbuvir
Ribavirin
PEG-IFNα 2a
Genotype
2
3
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
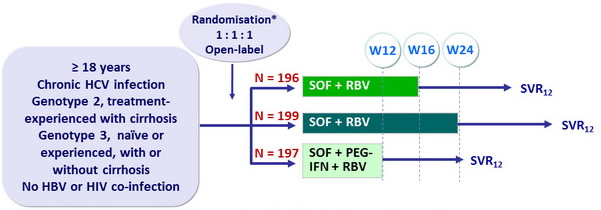
Design
*
Randomisation was stratified on genotype (2 or 3), prior therapy (yes or no) and cirrhosis (presence or absence)
- SOF 400 mg : 1 pill QD
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
- PEG-IFNα -2a : 180 mg SC once weekly
Objective
- SVR12 (HCV RNA < 15 IU/ml)
Baseline characteristics
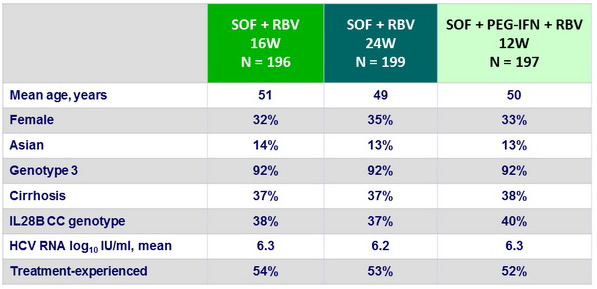
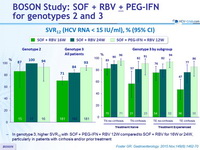
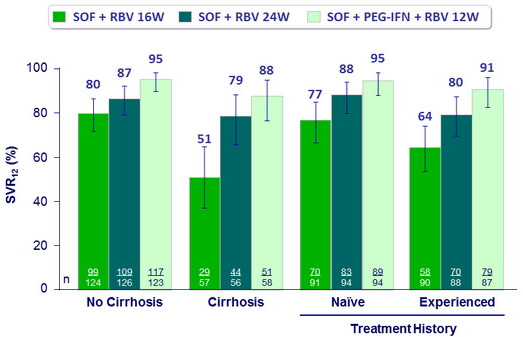
SVR12 (HCV RNA < 15 IU/ml), % (95% CI)
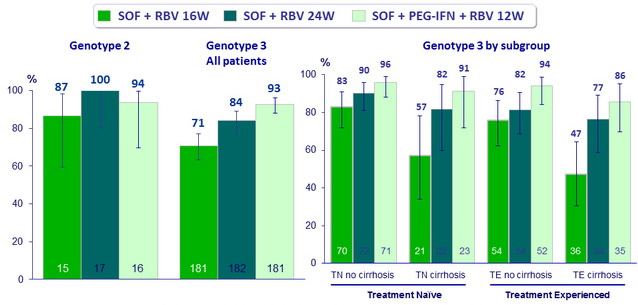
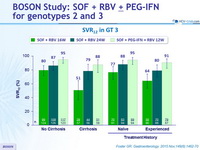
- In genotype 3, higher SVR12 with SOF + PEG-IFN + RBV 12W compared to SOF + RBV for 16W or 24W, particularly in patients with cirrhosis and/or prior treatment
SVR12 in GT3
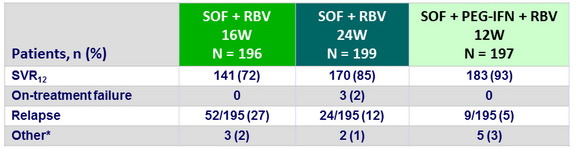
Reasons for not achieving SVR12
Resistance analysis
- Deep sequencing successful on 78/88 patients with virologic failure
- No S282T variants
- SOF treatment-emergent variants L159F and V321A in 9/78 (21 %)
- L159F at baseline and failure, N = 1, only at failure, N = 5
- V321A emerged at failure in 2 patients
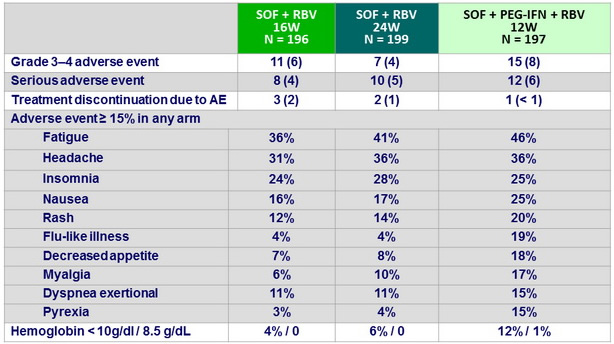
Adverse events, N (%)
Summary
- Genotype 2 : treatment-experienced patients with cirrhosis achieved high SVR12 rates with all regimens
- Genotype 3 : higher SVR12 rates with SOF + PEG/RBV than with SOF + RBV for 16 or 24 weeks
- Genotype 3 treatment-experienced patients with cirrhosis achieved an SVR12 of 86% with SOF + PEG + RBV for 12 weeks
- SOF + RBV for 24 weeks achieved SVR12 rates > 80% in all other subgroups; results consistent with earlier phase III studies
- SOF + RBV for 16 or 24 weeks and SOF + PEG + RBV for 12 weeks were well tolerated with a low rate of treatment discontinuations due to adverse events