AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis
Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44 ; Waked I, EASL 2016, Abs. SAT-166, J Hepatol 2016;64:S772
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Ribavirin
Genotype
4
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Design
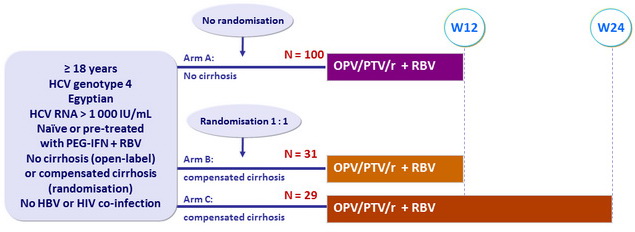
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg QD = 2 tablets
- RBV: 1000 mg/day if < 75 kg, 1200 mg/day if = 75 kg (bid dosing)
Objective
- SVR12 (HCV RNA < 15 IU/mL), with 2 sided 95% confidence interval, treatment-emergent adverse events
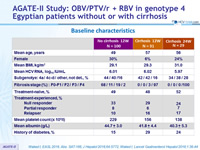
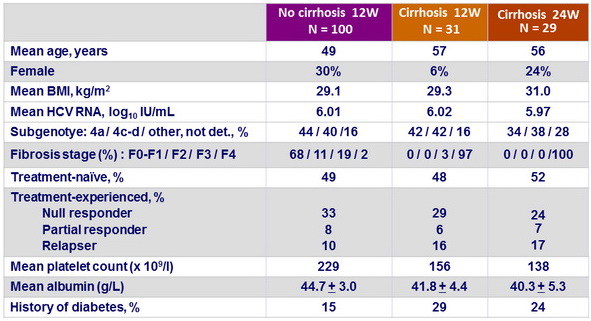
Baseline characteristics
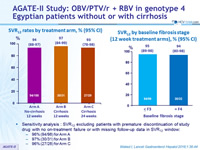
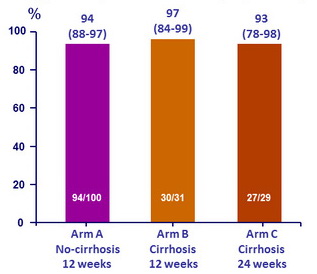
SVR rates by treatment arm, % (95% CI)
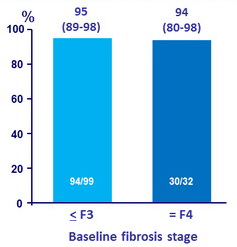
SVR12 by baseline fibrosis stage (12 week treatment arms), % (95% CI)
Sensitivity analysis : SVR12 excluding patients with premature discontinuation of study drug with no on-treatment failure or with missing follow-up data in SVR12 window :
- 96% (94/98) for Arm A
- 97% (30/31) for Arm B
- 96% (27/28) for Arm C
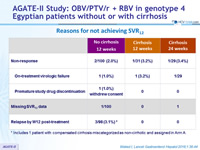
Reasons for not achieving SVR12
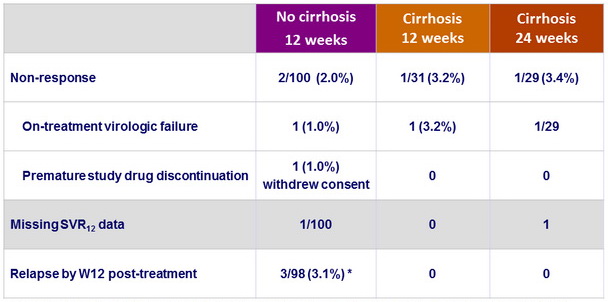
* Includes 1 patient with compensated cirrhosis miscategorized as non-cirrhotic and assigned in Arm A
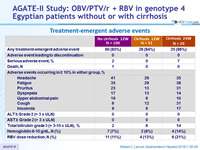
Treatment-emergence adverse events
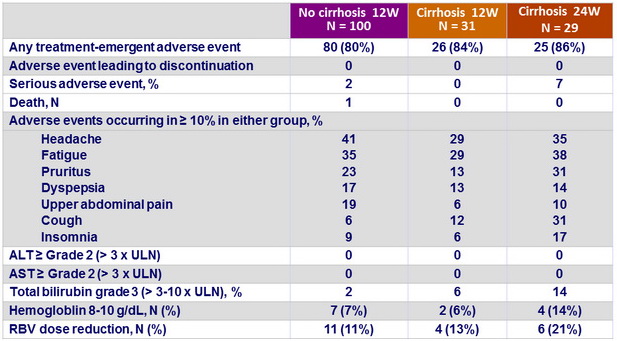
Summary
- High SVR rates were achieved in HCV genotype 4-infected Egyptian patients without cirrhosis or with compensated cirrhosis after 12 weeks of OBV/PTV/r + RBV weeks: SVR12 was 94% and 97%, respectively
- 12 weeks treatment was well tolerated with no treatment discontinuations due to adverse events
- Prolongation of therapy to 24 weeks does not provide additional benefit in patients with cirrhosis, with more serious adverse events and a higher frequency of hemoglobin decrease