ALLY-3+ study: DCV + SOF + RBV for HCV genotype 3 with advanced fibrosis or cirrhosis
ALLY-3+ study: DCV + SOF + RBV for HCV genotype 3 with advanced fibrosis or cirrhosis
Leroy V. Hepatology 2016; 63: 1430-41
Anti-HCV
Daclatasvir
Sofosbuvir
Ribavirin
Daclatasvir
Sofosbuvir
Ribavirin
Genotype
3
3
Treatment history
Naive
IFN-Experienced
SOF-experienced
Naive
IFN-Experienced
SOF-experienced
Cirrhosis
Yes
Yes
Design
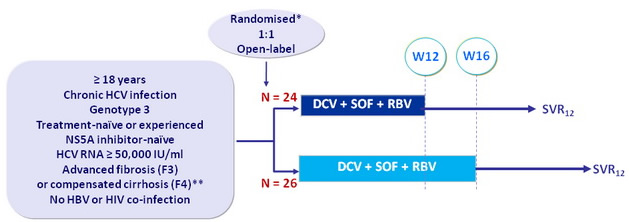
* Randomisation was stratified by fibrosis stage (F3 or F4)
** Liver biopsy or Fibroscan (= 9.6 to 14.6 = F3 ; > 14.6 kPa = F4), or Fibrotest ® = 0.75 + APRI > 2
- DCV : 60 mg qd
- SOF : 400 mg qd
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Objective
- SVR12 (HCV RNA < 25 IU/ml), with 95% CI, next observation carried backward
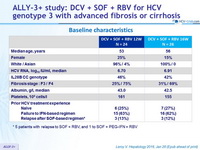
Baseline characteristics
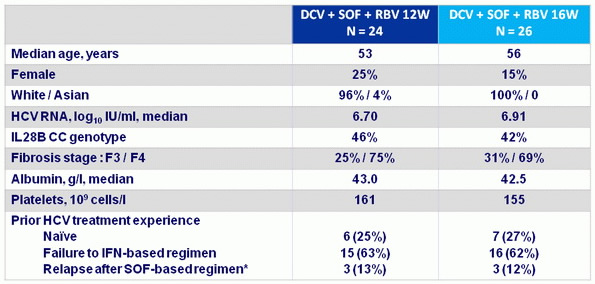
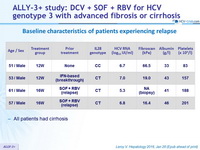
* 5 patients with relapse to SOF + RBV, and 1 to SOF + PEG-IFN + RBV
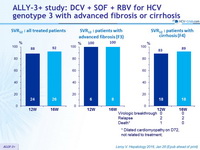
SVR12
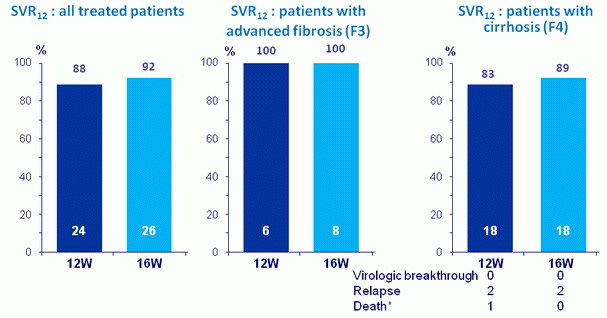
* Dilated cardiomyopathy on D72, not related to treatment;
Baseline characteristics of patients experiencing relapse
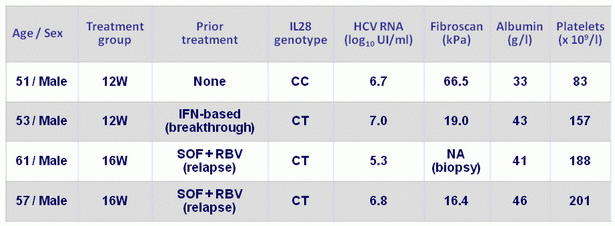
- All patients had cirrhosis
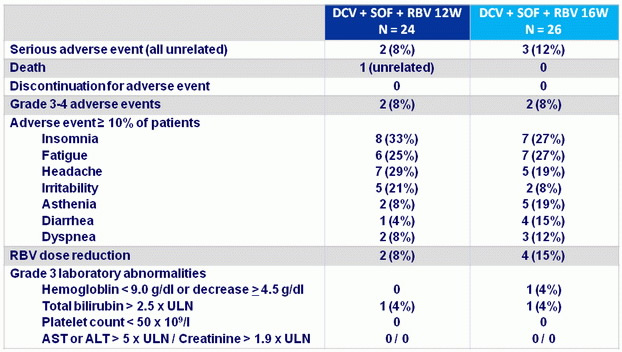
Adverse events
Summary
- Overall, 90% SVR 12 was achieved in HCV genotype 3- infected
patients with advanced fibrosis or compensated cirrhosis treated
with DCV + SOF + RBV
- SVR 12 was comparable for the 12-week ( 88%) and 16-week ( 92%) groups
- No on-treatment virologic failures; two relapses in each treatment arm
- 100% SVR 12 among patients with advanced fibrosis
- 86% SVR 12 among patients with cirrhosis (mostly treatment experienced)
- Treatment was safe and well tolerated; no patient discontinued for adverse event
- DCV + SOF + RBV for 12 or 16 weeks is a highly efficacious therapy for genotype 3-infected patients with advanced fibrosis or compensated cirrhosis