ASTRAL-1 study: SOF/VEL in genotype 1, 2, 4, 5 or 6
ASTRAL-1 study: SOF/VEL in genotype 1, 2, 4, 5 or 6
Feld JJ. N Engl J Med. 2015;373:2599-607; Younossi ZM, J. Hepatology 2016;65:33-9
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1
1a
1b
4
5
6
1
1a
1b
4
5
6
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
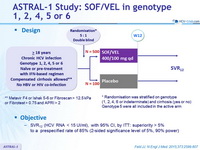
Design
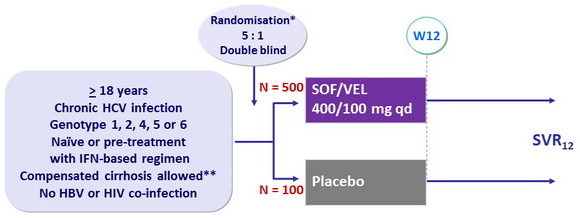
* Randomisation was stratified on genotype
(1, 2, 4, 6 or indeterminate) and cirrhosis (yes or no)
Genotype 5 were all included in the active arm
** Metavir F4 or Ishak 5-6 or Fibroscan > 12.5 kPa or Fibrotest > 0.75 and APRI > 2
Objective
- SVR12 (HCV RNA < 15 UI/ml), with 95% CI, by ITT: superiority > 5% to a prespecified rate of 85% (2-sided significance level of 5%, 90% power)
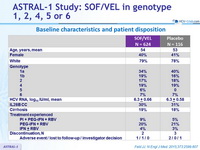
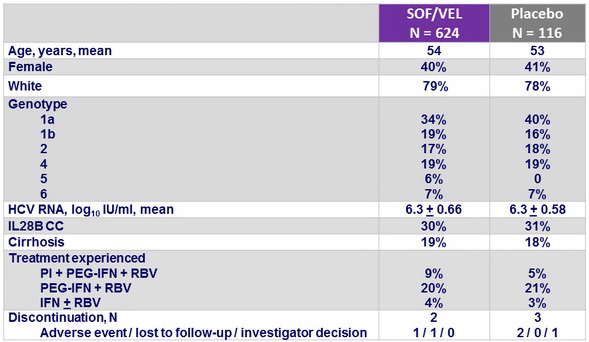
Baseline characteristics and patient disposition
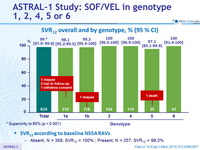
SVR12 overall and by genotype, % (95 % CI)
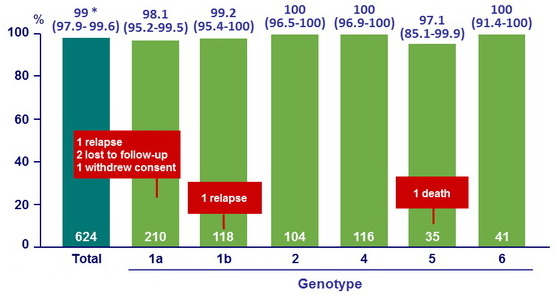
* Superiority to 85% (p < 0.001)
SVR12 according to baseline NS5A RAVs
- Absent, N = 359, SVR12 = 100% ; Present, N = 257, SVR12 = 99.2%
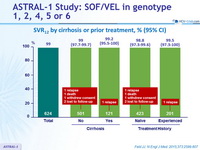
SVR12 by cirrhosis or prior treatment, % (95% CI)
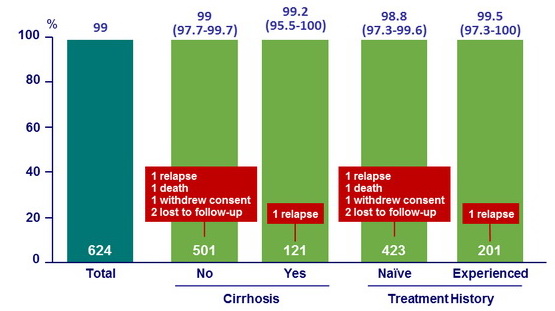
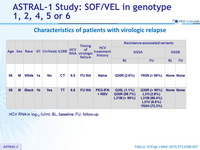
Characteristics of patients with virologic relapse
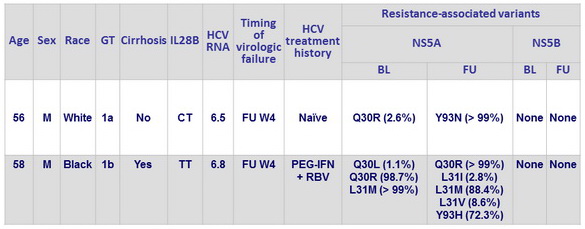
HCV RNA in log10 IU/ml; BL, baseline; FU, follow-up
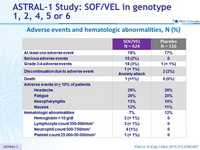
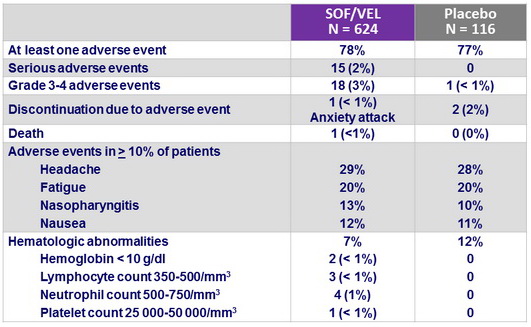
Adverse events and hematologic abnormalities, N (%)
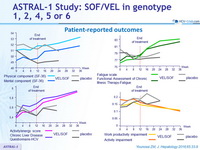
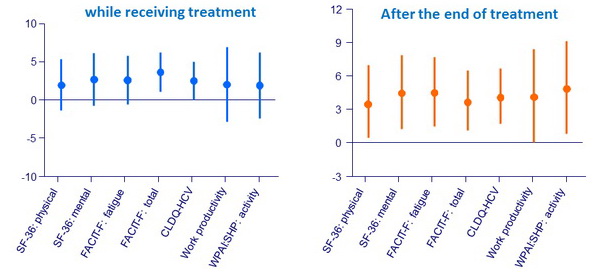
Patient-reported outcomes
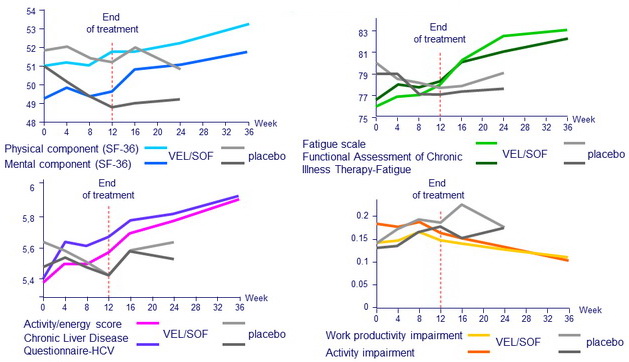
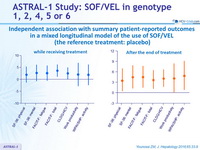
Independent association with summary patient-reported outcomes in a mixed longitudinal model of the use of SOF/VEL (the reference treatment: placebo)
Summary
- In this international, randomized, double-blind, placebo-controlled phase III study, treatment with sofosbuvir–velpatasvir for 12 weeks resulted in high rates of SVR12 (99%) in patients with HCV genotype 1, 2, 4, 5, or 6, including those with cirrhosis and those who had received previous treatment and those who had not been treated
- Virologic failure was rare in patients infected with HCV genotype 1,
and there were no virologic failures among those with HCV genotype 2, 4, 5 - Presence of baseline NS5A RAVs did not impact SVR12
- Although the 2 patients who had a relapse had RAVs at baseline and at the time of virologic failure, 99% of the patients with baseline NS5A RAVs had a SVR12 , which suggests that pretreatment testing for RAVs
is probably of little clinical value with SOF/VEL
- Although the 2 patients who had a relapse had RAVs at baseline and at the time of virologic failure, 99% of the patients with baseline NS5A RAVs had a SVR12 , which suggests that pretreatment testing for RAVs
- Treatment with SOF/VEL for 12 weeks was well tolerated, with a safety profile similar to that of placebo treatment
- SOF/VEL for 12 weeks provides a simple, safe, and highly effective treatment for patients with HCV GT 1, 2, 4, 5, or 6 infection, including those with compensated cirrhosis