ASTRAL-2 study: SOF/VEL vs SOF + RBV in genotype 2
ASTRAL-2 study: SOF/VEL vs SOF + RBV in genotype 2
Foster GR. N Engl J Med 2015; 373: 2608-17 & Sulkowki M. AASLD 2015; Abs. 205
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Genotype
2
2
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
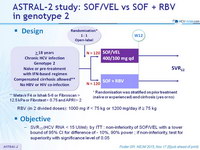
Design
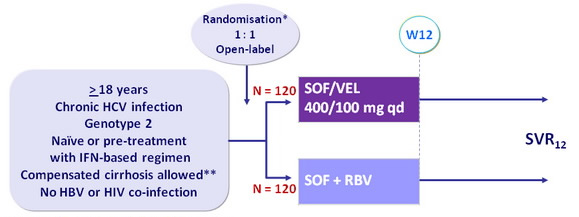
* Randomisation was stratified on prior treatment (naïve or experienced) and cirrhosis (yes or no)
** Metavir F4 or Ishak 5-6 or Fibroscan > 12.5 kPa or Fibrotest > 0.75 and APRI > 2
- RBV (in 2 divided doses) : 1000 mg if < 75 kg or 1200 mg/day if = 75 kg
Objective
- SVR12 (HCV RNA < 15 UI/ml) , by ITT : non-inferiority of SOF/VEL with a lower bound of 95% CI for difference of - 10%, 90% power ; if non-inferiority, test for superiority with significance level of 0.05
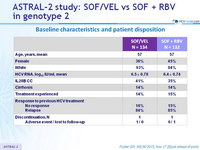
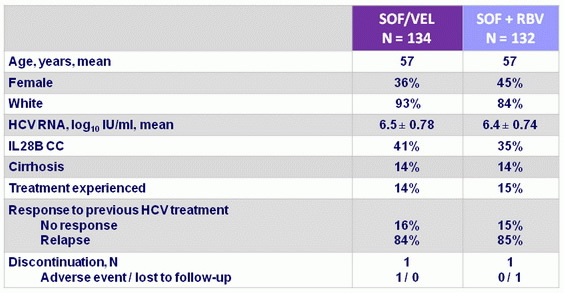
Baseline characteristics and patient disposition
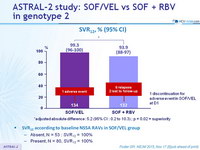
SVR12, % (95% CI)
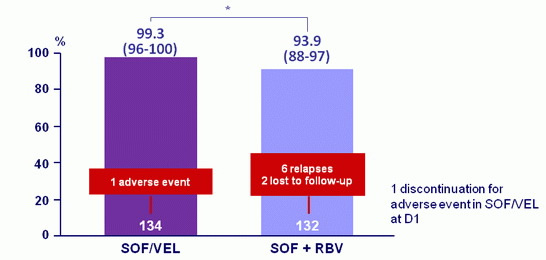
*adjusted absolute difference : 5.2 (95% CI : 0.2 to 10.3) ; p = 0.02 = superiority
SVR12 according to baseline NS5A RAVs in SOF/VEL group
- Absent, N = 53 : SVR12 = 100%
- Present, N = 80, SVR12 = 100%
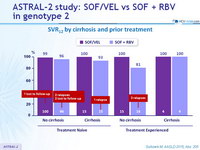
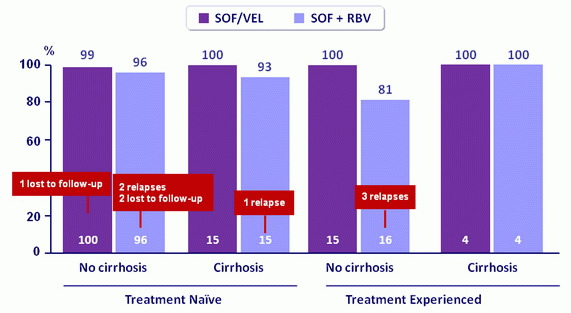
SVR12 by cirrhosis and prior treatment
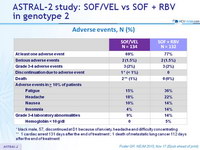
Adverse events, N (%)
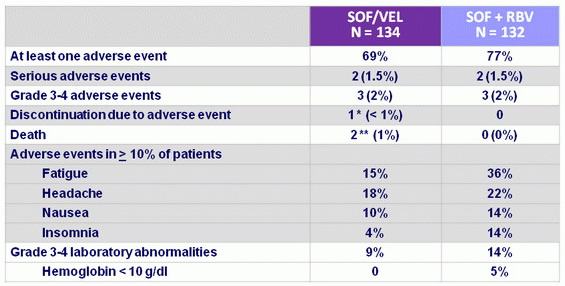
* black male, 57, discontinued at D1 because of anxiety, headache and difficulty concentrating
** 1 cardiac arrest 131 days after the end of treatment ; 1 death of metastatic lung cancer 112 days after the end of treatment
Summary
- Treatment with SOF/VEL for 12 weeks resulted in a 99 % SVR12 rate in patients with HCV genotype 2 infection
- SOF/VEL for 12 weeks was statistically superior to SOF + RBV for 12 weeks (p = 0.02)
- No patients who received SOF/VEL experienced virologic failure in a population that included patients with cirrhosis and previous treatment failure
- Limitation : small number of black patients
- Among patients with HCV genotype 2 who received SOF/VEL, the presence of baseline NS5A or NS5B RAVs was not associated with virologic failure
- SOF/VEL was well tolerated and, compared with SOF + RBV, lacked toxicities commonly associated with RBV
- SOF/VEL for 12 weeks provides a single tablet, once daily, highly effective, RBV-free treatment for patients with HCV genotype 2 infection