C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy
Dore GJ. Ann Intern Med 2016;165:625-636, EASL 2016, Abs. SAT-163, J Hepatol 2016;64:S771 & AASLD 2016, Abs. 871
Anti-HCV
Grazoprevir
Elbasvir
Grazoprevir
Elbasvir
Genotype
1a
1b
4
5
1a
1b
4
5
Treatment history
Naive
Naive
Cirrhosis
Yes
No
Yes
No
Special population
Opioid replacement
Opioid replacement
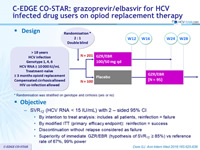
Design
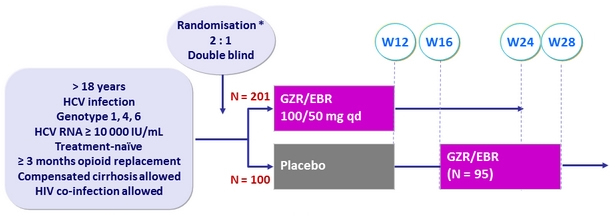
* Randomisation was stratified on genotype and cirrhosis (yes or no)
Objective
- SVR12 (HCV RNA < 15 IU/ml) with 2 – sided 95% CI
- By intention to treat analysis: includes all patients, reinfection = failure
- By modified ITT (primary efficacy endpoint): reinfection = success
- Discontinuation without relapse considered as failure
- Superiority of immediate GZR/EBR (hypothesis of SVR12 = 85%) vs reference rate of 67%, 99% power
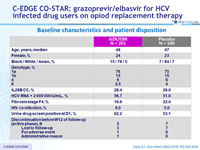
Baseline characteristics and patient disposition
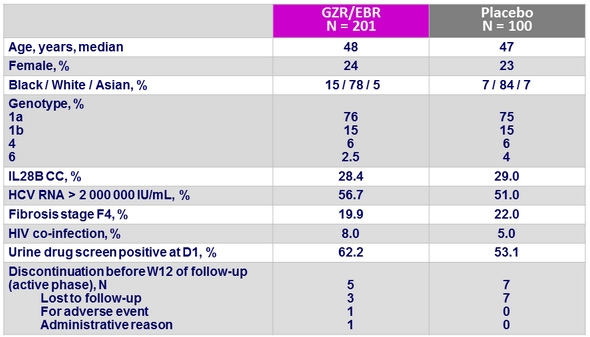
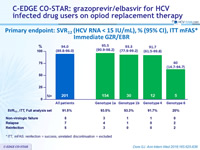
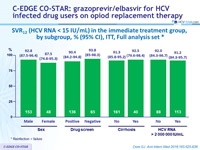
Primary endpoint : SVR12 (HCV RNA < 15 IU/ml), % (95% CI), ITT mFAS*
Immediate GZR/EBR
* ITT, mFAS : reinfection = success, unrelated discontinuation = excluded
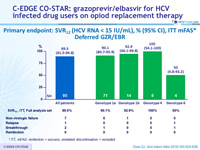
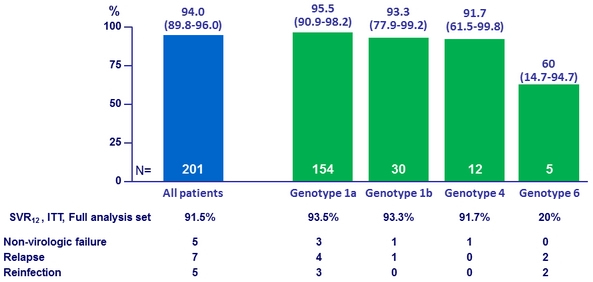
Primary endpoint : SVR12 (HCV RNA < 15 IU/ml), % (95% CI), ITT mFAS*
Deferred GZR/EBR
* ITT, mFAS : reinfection = success, unrelated discontinuation = excluded
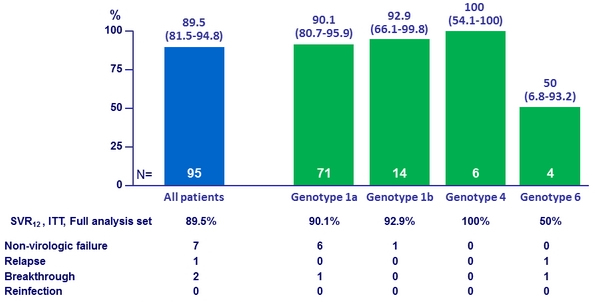
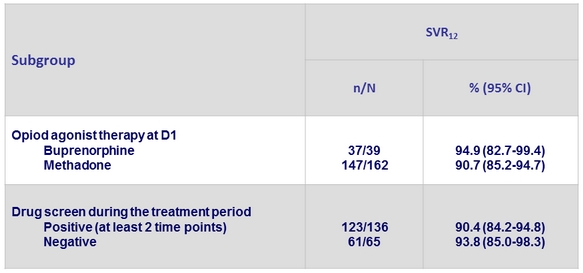
SVR12 (HCV RNA < 15 IU/ml) in the immediate treatment group, by subgroup, % (95% CI), ITT, Full analysis set *
* Reinfection = failure
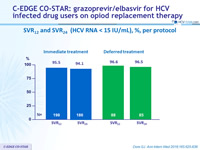
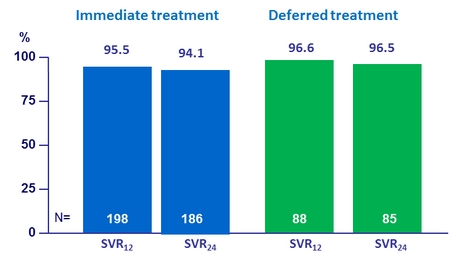
SVR12 and SVR24 (HCV RNA < 15 IU/ml), %, per protocol
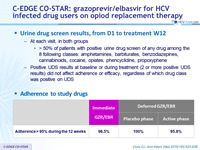
Urine drug screen results, from D1 to treatment W12
- At each visit, in both groups
- > 50% of patients with positive urine drug screen of any drug among the 8 following classes: amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine, propoxyphene
- Positive UDS results at baseline or during treatment (2 or more positive UDS results) did not affect adherence or efficacy, regardless of which drug class was positive on UDS
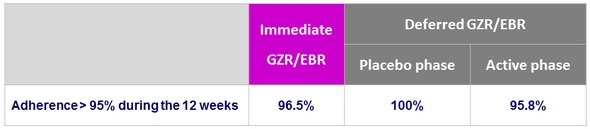
Adherence to study drugs
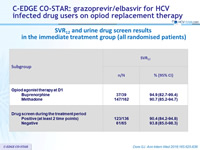
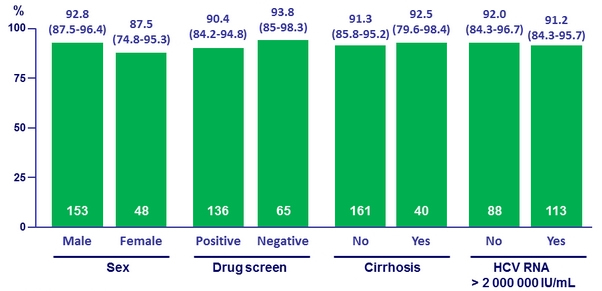
SVR12 and urine drug screen results in the immediate treatment group (all randomised patients)
Reinfection
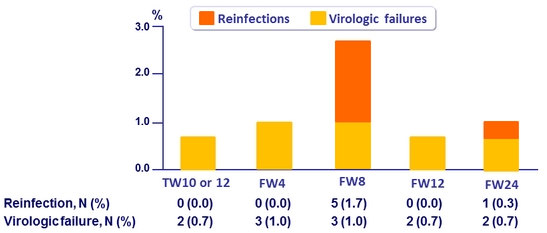
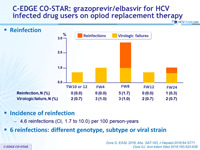
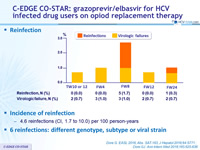
Incidence of reinfection
- 4.6 reinfections (CI, 1.7 to 10.0) per 100 person-years
- 6 reinfections : different genotype, subtype or viral strain
Summary of 18 patients with virological failure or probable reinfection
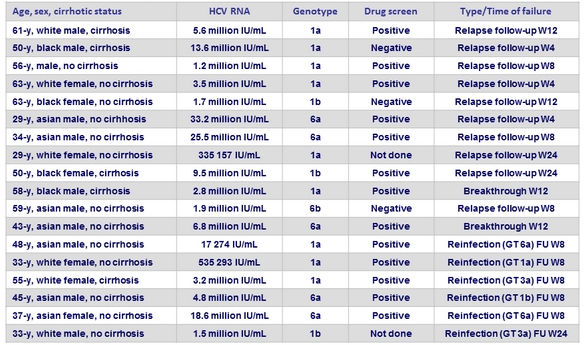
Reinfection during post-treatment and follow-up (3 years post-treatment)
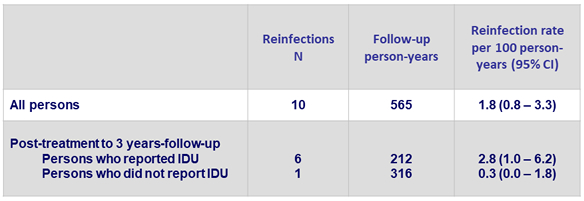
IDU = intravenous drug use
Reinfection
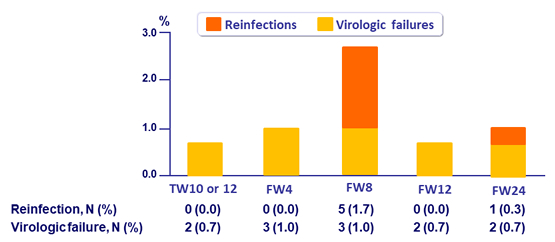
- Incidence of reinfection
- 4.6 reinfections (CI, 1.7 to 10.0) per 100 person-years
- 6 reinfections: different genotype, subtype or viral strain
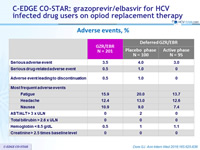
Adverse events, %

Summary
- EBR/GZR demonstrated high efficacy in genotype 1
and 4-infected patients receiving opiate agonist therapy
- Limitation: small number of genotype 6-infected patients
- Acceptable safety profile with comparable adverse event rates between the immediate and deferred treatment arms
- High study medication adherence
- Stable ongoing drug use throughout the initial treatment phase in both groups
- Reinfection rate is higher in the immediate follow-up period
- These results support the removal of drug use as a barrier to interferon-free HCV treatment for patients receiving opiate agonist therapy
3-year Follow-up on Risk Factors and Rate of Reinfection
- Observational cohort (6-month follow-up visits)
- Follow-up of 199/296 (67%) patients enrolled in Co-STAR
- Positive urine drug screen (UDS) at enrollment: 56% and 58% of patients in the 3YFU study and the Phase 3 trial, respectively
- Median time from EOT to the first visit during the 3YFU was 330 days (range: 206-485)
- 84 (56%) patients reported any drug use (non-injecting or injecting) in the past 6 months. Injecting drug use in the past 6 months was reported by 25% of patients
- Reinfection rate : higher in the immediate follow-up period through week (FW)12 (N = 5) than in the period through FW24 (+1) and through the ongoing observational follow-up
- Overall reinfection rate through the 6-month follow-up period is 4.0/100 person-years ( N = 8) [ 95% CI : 1.7-8.0]
- Including only those patients with persistence of viremia (N = 5), the effective reinfection rate is 2.5/100 person-years [95% CI : 0.8-5.9]
- These data support addressing barriers in the treatment of patients on opiate agonist therapy and patients with ongoing drug use