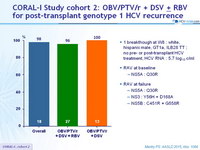
CORAL-I Study cohort 2: OBV/PTV/r + DSV ± RBV for post-transplant genotype 1 HCV recurrence
CORAL-I Study cohort 2: OBV/PTV/r + DSV ± RBV for post-transplant genotype 1 HCV recurrence
Mantry PS. AASLD 2015, Abs. 1084
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Cirrhosis
No
No
Special population
Liver transplantation
Liver transplantation
Design
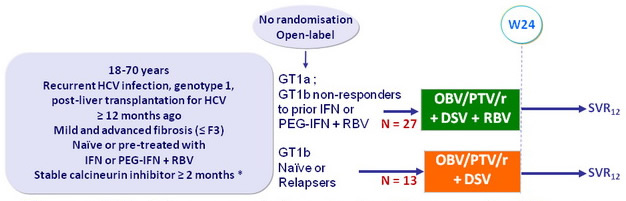
* Recommended dose for immunosuppressive therapy: tacrolimus : 0.5 mg once weekly or 0.2 mg every 3 days, cyclosporine: one-fifth of the daily pre-study dose qd ; dosing subsequently guided by TDM
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV): 250 mg bid
- RBV: dose selected by investigator (most often 600-800 mg/day)
Objective
- SVR12 (HCV RNA < 25 IU/ml), with 95% CI, by ITT, descriptive analysis
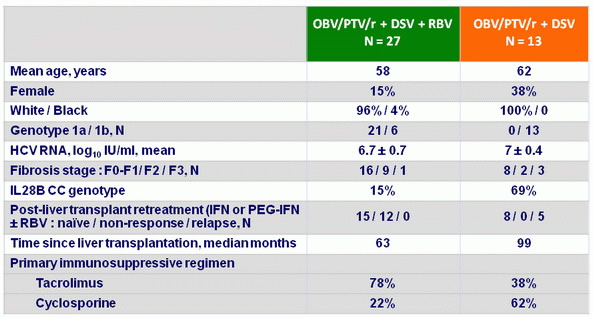
Baseline characteristics
* At relapse : resistance-associated variants R155K in NS3, M28T and Q30R in NS5A, and G554S in NS5B
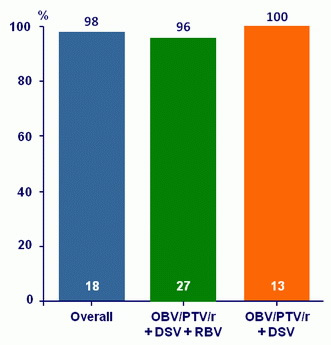
- 1 breakthough at W8 : white, hispanic male, GT1a, ILB28 TT ; no pre- or post-transplant HCV treatment, HCV RNA : 5.7 log10 c/ml
- RAV at baseline
- NS5A : Q30R
- RAV at failure
- NS5A : Q30R
- NS3 : Y56H + D168A
- NS5B : C451R + G558R
Adverse events
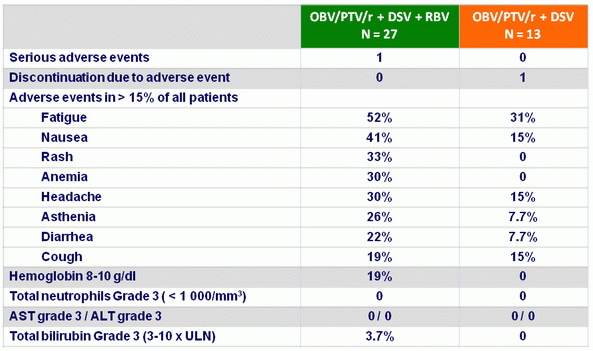
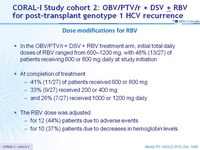
Dose modifications for RBV
- In the OBV/PTV/r + DSV + RBV treatment arm, initial total daily doses of RBV ranged from 600–1200 mg, with 48% (13/27) of patients receiving 600 or 800 mg daily at study initiation
- At completion of treatment
- 41% (11/27) of patients received 600 or 800 mg
- 33 % (9/27) received 200 or 400 mg
- and 26% (7/27) received 1000 or 1200 mg daily
- The RBV dose was adjusted
- for 12 (44%) patients due to adverse events
- for 10 (37%) patients due to decreases in hemoglobin levels
Summary
- In cohort 2 of the CORAL-I study, adult liver transplant recipients with recurrent HCV genotype 1 and mild to advanced fibrosis achieved high SVR12 rates with the regimen of OBV/PTV/r + DSV ± RBV
- Treatment was generally well tolerated and immunosuppresive drugs (calcineurin inhibitors) dosing was manageable over the period of the study
- The RBV-free regimen of OBV/PTV/r + DSV achieved 100% SVR12 in patients with GT1b infection and will be implemented in future cohort