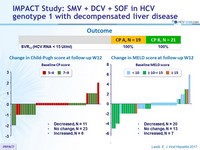
IMPACT Study: SMV + DCV + SOF in HCV genotype 1 with decompensated liver disease
IMPACT Study: SMV + DCV + SOF in HCV genotype 1 with decompensated liver disease
Lawitz E. J Viral Hepat 2017; 24:287-94
Anti-HCV
Simeprevir
Daclatasvir
Sofosbuvir
Simeprevir
Daclatasvir
Sofosbuvir
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
Yes
Special population
Decompensated liver disease
Decompensated liver disease
Design
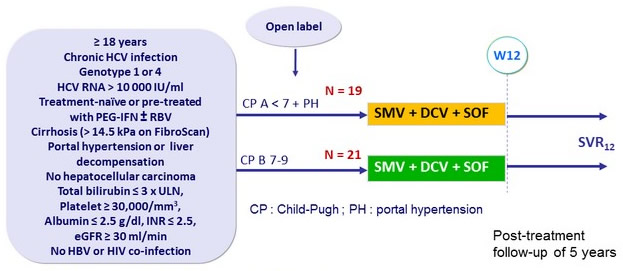
- SMV 150 mg qd + DCV 90 mg qd + SOF 400 mg qd
Objective
- SVR12 (HCV RNA < 15 IU/ml), with 2-sided 95% CI, by ITT
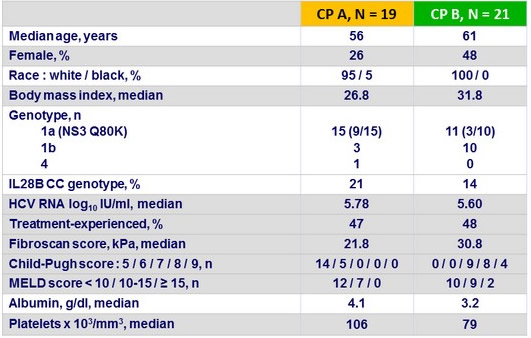
Baseline characteristics
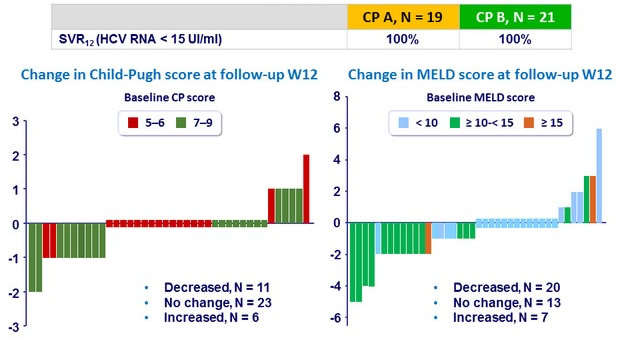
Outcome
SMV, DCV and SOF pharmacokinetics following administration of SMV + DCV + SOF
- Intensive PK analysis performed at Week 2 and Week 8
- Mean SMV exposure: 2.2-fold higher in CP B than CP A
- Mean DCV exposure: similar in CP B and CP A
- Mean SOF exposure: 1.4-fold higher in CP B than CP A
- Mean GS-331007 exposure (SOF metabolite): similar in CP B and CP A
Adverse events and laboratory abnormalities, N (%)
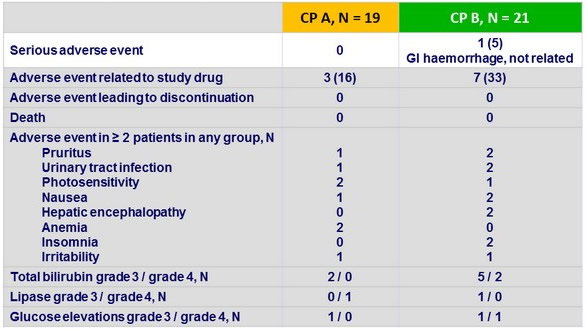
Summary
- Treatment for 12 weeks with SMV, SOF, and DCV resulted in 100% response rate; all 19 Child-Pugh A patients and all 21 Child-Pugh B patients achieved SVR12
- High virologic response was observed regardless
- of Child-Pugh class (<7 or 7–9)
- or the presence of resistance-associated variants at baseline
- This 3-DAA combination was generally safe and well tolerated
- No discontinuations due to adverse events
- No deaths
- 5-year follow-up will be important in providing long-term outcomes in association with SVR