C-EDGE co-infection study: grazoprevir / elbasvir in HIV coinfection
Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial
Rockstroh JK. Lancet HIV 2015;2:e319-e327
Anti-HCV
Grazoprevir
Elbasvir
Grazoprevir
Elbasvir
Genotype
1
1a
1b
4
1
1a
1b
4
Treatment history
Naive
Naive
Special population
HIV co-infection
HIV co-infection
Design
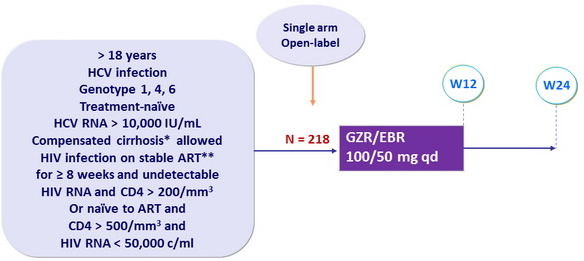
*
Metavir F4 or F ibroscan > 12.5 kPa or FibroTest > 0.75 + APRI > 2
**
TDF or ABC + 3TC or FTC + RAL or DTG or RPV
Objective
- SVR12 (HCV RNA < 15 IU/ mL ) by intention to treat analysis , 99% power to establish superiority over historical reference rate of 70% (PHOTON-1 Study ), with 1-sided 2.5% alpha level
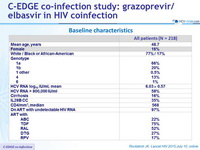
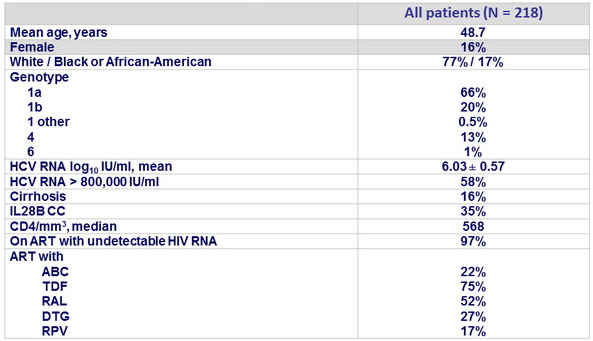
Baseline characteristics
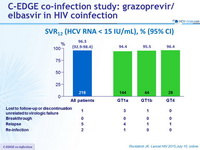
SVR12 (HCV RNA < 15 IU/ mL), % (95% CI)
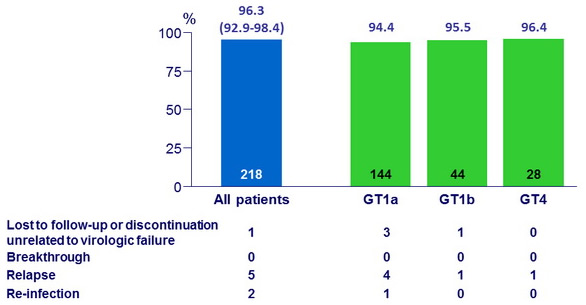
SVR12 by subgroups
- No differences for key subgroups characteristics (gender, age, race, IL28B genotype, cirrhosis, baseline HCV RNA, RAL vs DTG vs RPV)
- ABC-containing : SVR12 = 93.6% vs TDF-containing : SVR12 = 97.6%
Relapse, n = 5
- All non-cirrhotic, g enotype 1a, N = 4 ; g enotype 4, N = 1
- 4/5 were receiving ART : TDF-based ART N = 3 ; ABC-based ART N = 1
Re-infection, n = 2
- The 2 patients were infected with a different HCV genotype during follow-up compared with baseline : Genotype 1a and Genotype 1b at enrolment ; Genotype 3 at f ollow-up W12 in both
- Per protocol, these patients were classified as a failure for analysis, but sequencing data are consistent with post-treatment re-infection
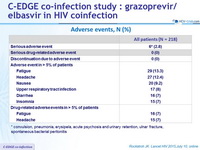
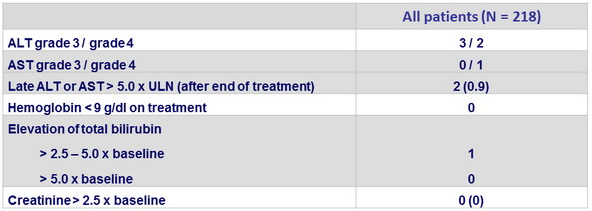
Adverse events, N (%)
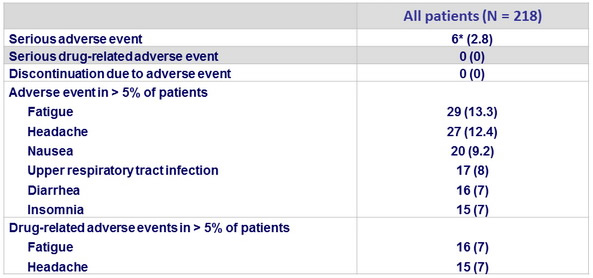
* convulsion, pneumonia , erysipela , acute psychosis and urinary retention , ulnar fracture, spontaneous bacterial peritonitis
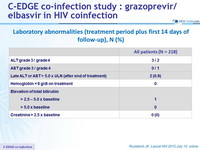
Laboratory abnormalities (treatment period plus first 14 days of follow-up), N (%)
Summary
- A 12-week regimen of the oral fixed dose combination of once-daily, single-tablet of grazoprevir/elbasvir, achieved an overall SVR12 of 96% in patients with HCV genotype 1 or 4 infection and HCV co-infection
- Comparable response rates to HCV mono-infected genotype 1 and 4
- Comparable SVR across all patients sub-groups
- Low rate of adverse events
- HIV breakthrough
- 2 patients with transient viremia and subsequent re-suppression with no change in ARV regimen
- No change in CD4+ cell counts
- Limitation
- No active-control group