RUBY-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir ± RBV for HCV genotype 1 with renal impairment
RUBY-I Study: ombitasvir/paritaprevir/ritonavir + dasabuvir ± RBV for HCV genotype 1 with renal impairment
Pockros PJ. Gastroenterology 2016 ; 150:1590-8
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Special population
Chronic Kidney disease
Chronic Kidney disease
Design
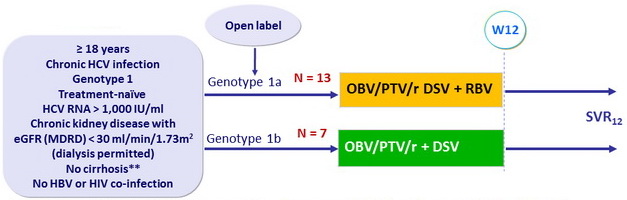
** Liver biopsy ( Metavir = F3, Ishak score = 4) or fibroscan < 12.5 kPa or FibroTest = 0.72 + APRI = 2
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV): 250 mg bid
- RBV 200 mg qd (genotype 1a)
Objective
- SVR12 ( HCV RNA < 25 IU/ml ) by intent-to-treat with 2-sided 95% CI
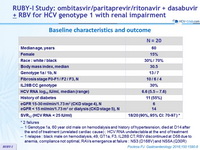
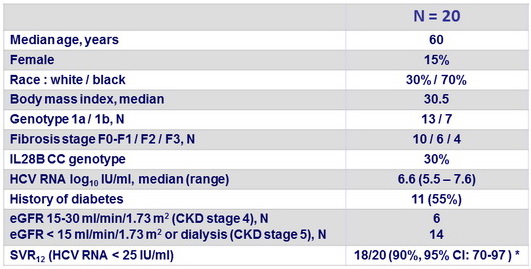
Baseline characteristics and outcome
* 2 failures
- 1 Genotype 1a, 60 year old male on hemodialysis and history of hypertesnsion , died at D14 after the end of treatment (unrelated cardiac cause) ; HCV RNA undetectable at the end of treatment
- 1 relapse : black male on hemodialysis, 49, GT1a, F3, IL28B CT, RBV discontinued at D58 due to anemia, compliance not optimal, RAVs emergence at failure : NS3 (D168V) and NS5A (Q30R)
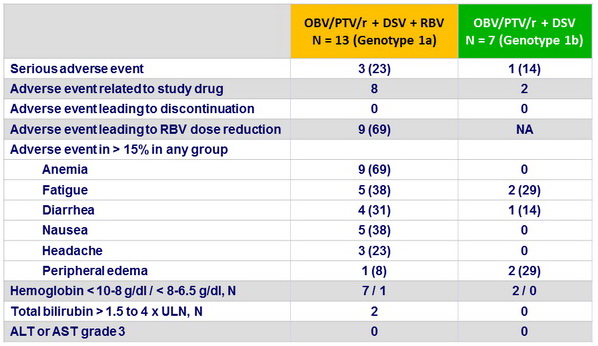
Adverse events and laboratory abnormalities, N (%)
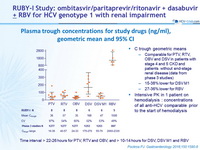
Plasma trough concentrations for study drugs (ng/ml), geometric mean and 95% CI
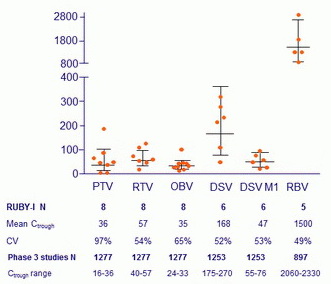
Time interval > 22-26 hours for PTV, RTV and OBV, and > 10-14 hours for DSV, DSV M1 and RBV
- C trough geometric means
- Comparable for PTV, RTV, OBV and DSV in patients with stage 4 and 5 CKD and patients without end-stage renal disease (data from phase 3 studies)
- 15-38% lower for DSV M1
- 27-36% lower for RBV
- Intensive PK in 1 patient on hemodialysis : concentrations of all anti-HCV comparable prior to the start of hemodialysis
Summary
- In HCV genotype 1-infected patients with stage 4 or 5 chronic kidney disease, including patients on dialysis, 12 weeks of therapy achieved a SVR12 of 90% overall
- Among patients with post-treatment data available, the SVR12 rate was
- For genotype 1a : 92.3% (12/13) with OBV/PTV/r + DSV + RBV ; 1 relapse, possibly related to poor treatment compliance
- For genotype 1b : 100% (7/7) with OBV/PTV/r + DSV
- OBV/PTV/r + DSV ± RBV was well tolerated with no treatment discontinuations
- The majority of patients receiving RBV 200 mg/day required RBV interruption (9/13)
- Laboratory abnormalities and safety findings were otherwise consistent with the safety profile in patients with normal renal function
- These data support the use of the 3D regimen with no dose adjustment in patients with severe or end-stage renal disease