SYNERGY-D Study: LDV/SOF retreatment in genotype 1 failing short-course LDV/SOF
SYNERGY-D Study: LDV/SOF retreatment in genotype 1 failing short-course LDV/SOF
Wilson EM. Clin Infect Dis 2016; 62:230-8
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1a
1
1a
Treatment history
NS5A experienced
SOF-experienced
NS5A experienced
SOF-experienced
Cirrhosis
No
No
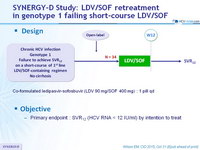
Design
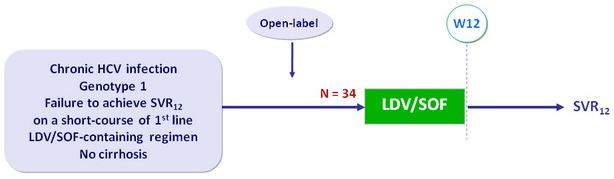
- Co-formulated ledipasvir-sofosbuvir (LDV 90 mg/SOF 400 mg) : 1 pill qd
Objective
- Primary endpoint : SVR12 (HCV RNA < 12 IU/ml) by intention to treat
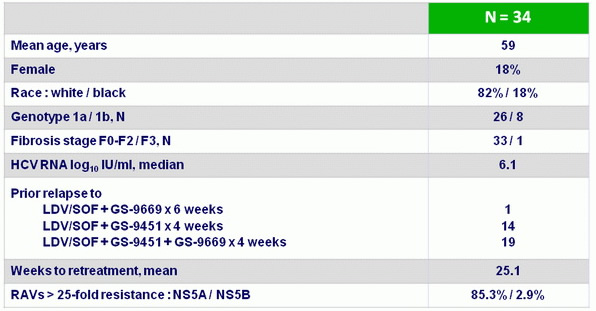
Baseline characteristics
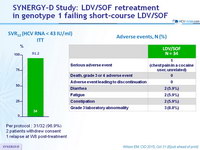
SVR12 (HCV RNA < 43 IU/ml) - ITT
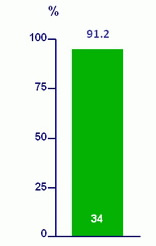
Per protocol : 31/32 (96.9%)
2 patients withdrew consent
1 relapse at W8 post-treatment
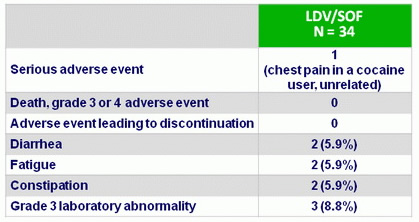
A dverse events , N (%)
Deep sequencing at baseline (before 1 st LDV/SOF treatment), at relapse, and prior to retreatment
Prior to retreatment
- NS5A RAVs : 29/34 : SVR 12 in 26/29 (89.7%) [ITT] ; 96.3% per protocol
- RAVs with > 100 fold resistance to LDV
- L31M/V/I, N = 17 (L31M, N = 16)
- Q30R/H/T, N = 13 (Q30R, N = 8)
- Y93H/C/N, N = 7 (Y93H, N = 6)
- RAVs with > 100 fold resistance to LDV
- NS5B RAV : 1/34 : 1/1 achieved SVR 12
- L159F
Relapse (n=1)
- Genotype 1b, 1st treatment with LDV/SOF + GS-9451 x 6 weeks. RAV at baseline = L31M, at 1 st relapse and prior to retreatment : L31M + Y93H, no NS5B mutant. At failure (relapse of retreatment) : emergence of S282T + V321I (NS5B), no additional NS5A RAVs
Summary
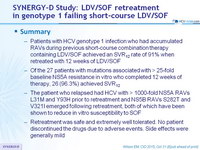
- Patients with HCV genotype 1 infection who had accumulated RAVs during previous short-course combination therapy containing LDV/SOF achieved an SVR12 rate of 91% when retreated with 12 weeks of LDV/SOF
- Of the 27 patients with mutations associated with > 25-fold baseline NS5A resistance in vitro who completed 12 weeks of therapy, 26 (96.3%) achieved SVR12
- The patient who relapsed had HCV with > 1000-fold NS5A RAVs L31M and Y93H prior to retreatment and NS5B RAVs S282T and V321I emerged following retreatment, both of which have been shown to reduce in vitro susceptibility to SOF
- Retreatment was safe and extremely well tolerated. No patient discontinued the drugs due to adverse events. Side effects were generally mild