TOPAZ-II Study: OBV/PTV/r + DSV ± RBV for genotype 1
TOPAZ-II Study: OBV/PTV/r + DSV ± RBV for genotype 1
Neau R. AASLD 2015, Abs. 1065
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Design
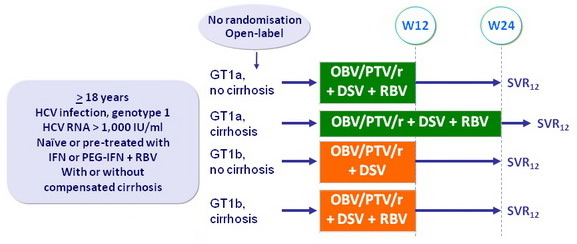
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV): 250 mg bid
- RBV: 1 000 or 1 200 mg/day in 2 doses according to body weight (< or > 75 kg)
Objective
- SVR12 (HCV RNA <15 IU/ml), with 95% CI, by ITT, descriptive analysis
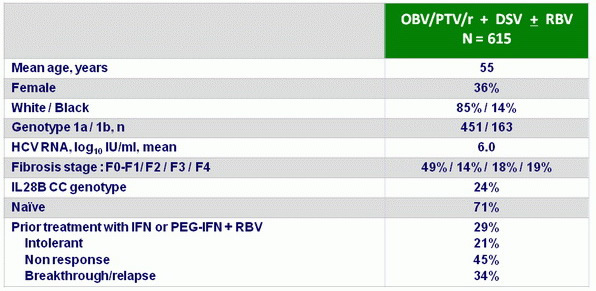
Baseline characteristics
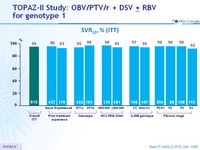
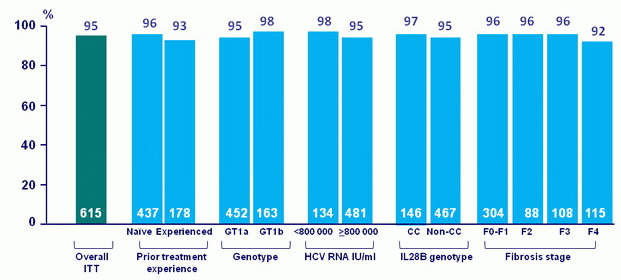
SVR12, % (ITT)
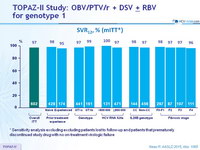
SVR12, % (mITT*)
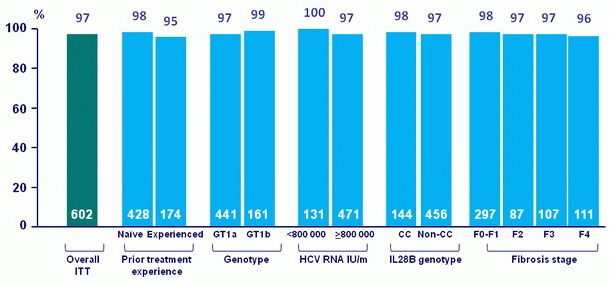
* Sensitivity analysis excluding excluding patients lost to follow-up and patients that prematurely discontinued study drug with no on-treatment virologic failure
Patients not achieving SVR12 (ITT population)
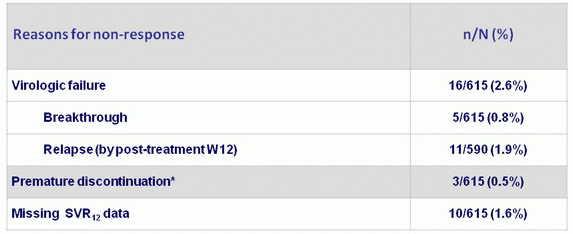
*2 patients discontinued due to an adverse event ; 1 patient withdrew consent
Treatment emerging adverse events and laboratory abnormalities, N (%)
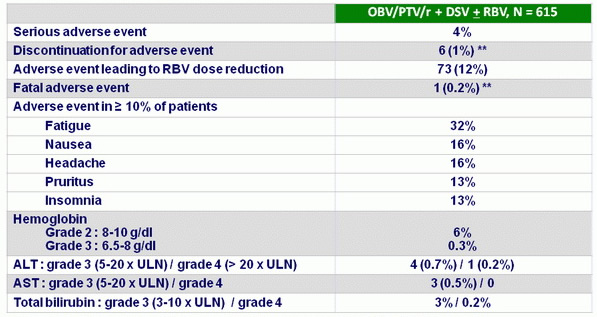
* Nausea (N = 2), fatigue (N = 1), lethargy/vomiting (N = 1), hypercortisolism (N = 1), peritonitis (N = 1)
** Death due to metastatic pancreatic adenocarcinoma (Stage IV). Unrelated to HCV therapy
Summary
- In the TOPAZ-II study, treatment-naïve or -experienced patients with HCV genotype 1 infection with or without cirrhosis achieved high SVR12 rates (95.3% in the ITT population and 97.3% in the mITT population) with 12- or 24-weeks of treatment with OBV/PTV/r + DSV ± RBV
- Treatment was well tolerated with low rates of serious adverse events and study drug discontinuations due to adverse events