C-CREST study, Part A: GZR + EBR or MK-8408 + MK-3682 for genotypes 1, 2 and 3 - Phase II
Gane EJ. Lancet Gastroenterol Hepatol 2017 ; 2 :805-813
Anti-HCV
Grazoprevir
Elbasvir
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Grazoprevir
Elbasvir
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Genotype
1
2
3
1
2
3
Treatment history
Naive
Naive
Cirrhosis
No
No
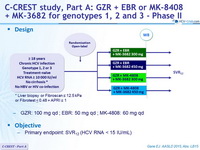
Design
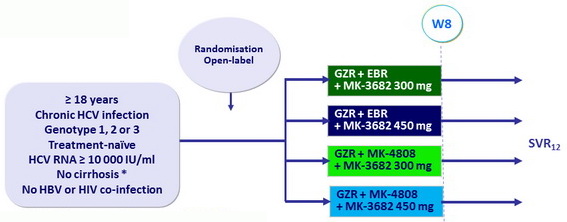
* Liver biopsy or Fibroscan ≤ 12.5 kPa or Fibrotest < 0.48 + APRI ≤ 1
- GZR: 100 mg qd ; EBR: 50 mg qd ; MK-4808: 60 mg qd
Objective
- Primary endpoint: SVR12 (HCV RNA < 15 IU/ml)
Baseline characteristics
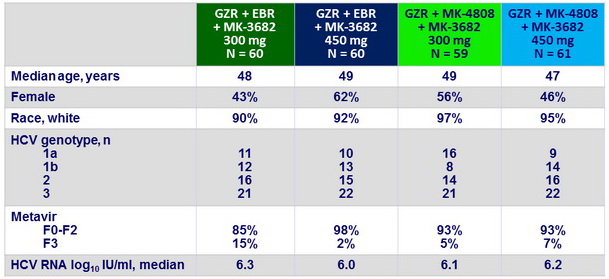
- All 240 enrolled patients completed 8 weeks of treatment and reached follow-up W12 post-treatment
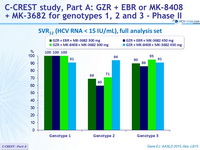
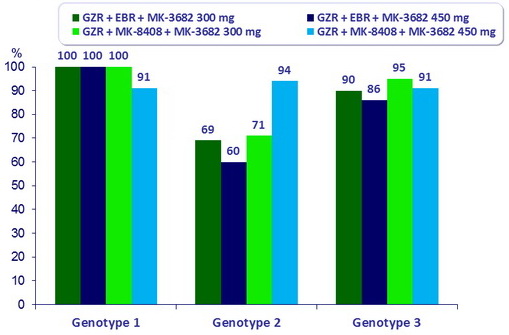
SRV12 (HCV RNA < 15 IU/ml), full analysis set
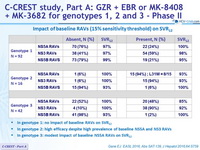
Impact of baseline NS5A RAVs
- No impact of baseline genotype 1 NS5A RAVs on SVR12
- SVR12 : 97% if no RAVs, 100% if RAVs
- No treatment emergent NS5A RAVs in the 2 relapses (1 genotype 1a and 1 genotype 1b)
- High SVR12 in genotype 3 with GZR + MK-4808 + MK-3862 despite high prevalence (47%) of NS5A RAVs
- SVR12 : 100% if no RAVs, 85% if RAVs
- Treatment-emergent NS5A RAV in 1 of the 3 relapses (Y93H)
Impact of baseline RAVs (15% sensitivity threshold) on SRV12
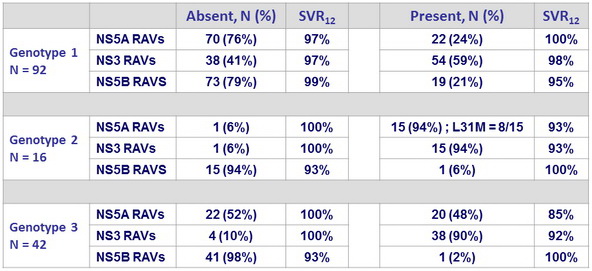
- In genotype 1: no impact of baseline RAVs on SVR12
- In genotype 2: high efficacy despite high prevalence of baseline NS5A and NS3 RAVs
- In genotype 3: modest impact of baseline NS5A RAVs on SVR12
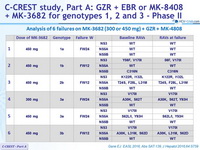
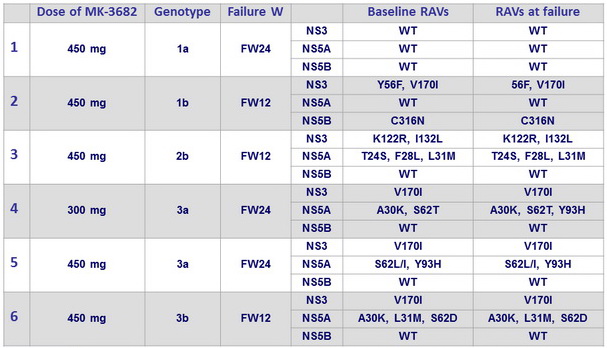
Analysis of 6 failures on MK-3682 (300 or 450 mg) + GZR + MK-4808
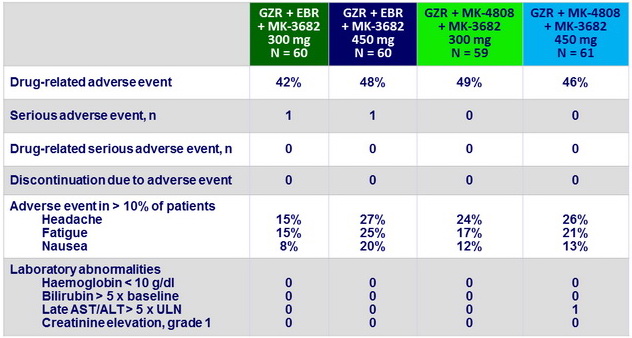
Adverse events
Summary
- In this pilot phase II, randomised , open-label study, treatment with GZR + MK-4808 + MK36-82 was well tolerated and resulted in high rates of SVR12 (91–94%) in treatment-naïve non-cirrhotic patients with genotypes 1, 2 or 3
- Improved SVR12 (85%) in patients with genotype 3 and baseline NS5A RAVs
- Good safety and tolerability
- GZR/MK-4808/MK3682 (450 mg) selected for study Part B