LEAGUE-1 study: daclatasvir + SMV ± RBV for genotype 1
Zeuzem S, J Hepatol 2016; 64:292-300
Anti-HCV
Simeprevir
Daclatasvir
Ribavirin
Simeprevir
Daclatasvir
Ribavirin
Genotype
1a
1b
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
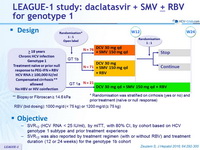
Design
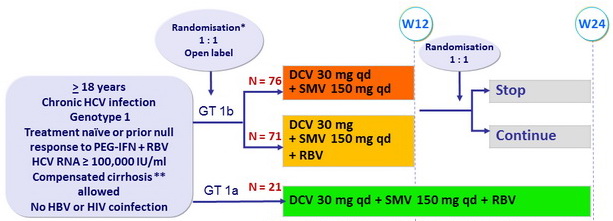
* Randomisation was stratified on cirrhosis (yes or no) and prior treatment (naïve or null response)
** Biopsy or Fibroscan = 14.6 kPa
- RBV (bid dosing): 1000 mg/d (< 75 kg) or 1200 mg/d (= 75 kg)
Objective
- SVR12 (HCV RNA < 25 IU/ml), by mITT , with 80% CI, by cohort based on HCV genotype 1 subtype and prior treatment experience
- SVR12 was also reported by treatment regimen (with or without RBV) and treatment duration (12 or 24 weeks) for the genotype 1b cohort
Baseline characteristics and patient disposition
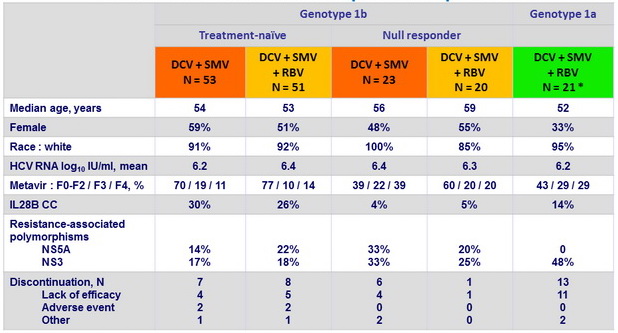
* Treatment-naïve, N = 12 ; null responder to prior PEG-IFN + RBV, N = 9
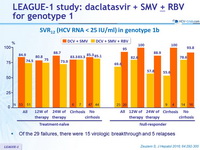
SRV12 (HCV RNA < 25 IU/ml) in genotype 1b)
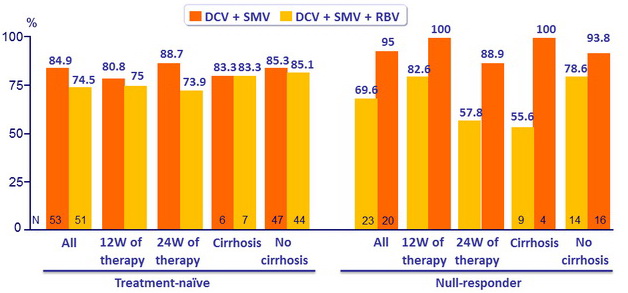
- Of the 29 failures, there were 15 virologic breakthrough and 5 relapses
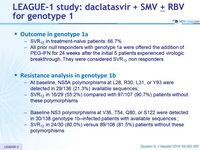
Outcome in genotype 1a
- SVR12 in treatment-naïve patients: 66.7%
- All prior null responders with genotype 1a were offered the addition of PEG-IFN for 24 weeks after the initial 5 patients experienced virologic breakthrough. They were considered SVR12 non responders
Resistance analysis in genotype 1b
- At baseline, NS5A polymorphisms at L28, R30, L31, or Y93 were detected in 29/136 (21.3%) available sequences;
- SVR12 in 16/29 ( 55.2%) compared with 97/107 (90.7%) patients without these polymorphisms
- Baseline NS3 polymorphisms at V36, T54, Q80, or S122 were detected in 30/138 genotype 1b –infected patients with available sequences ;
- SVR12 in 24/30 (80.0%) versus 89/108 (81.5% ) patients without these polymorphisms
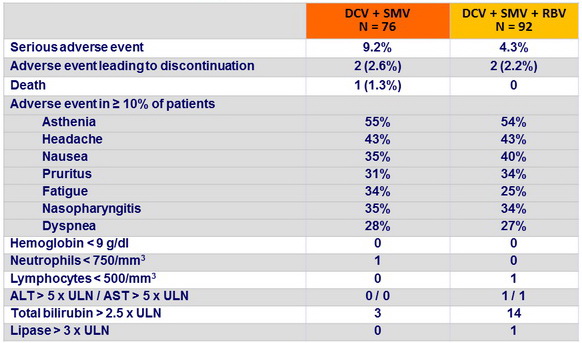
Adverse events and grade 3-4 laboratory abnormalities, N (%)
Summary
- With all -oral, IFN- free treatment of DCV + SMV, with or without RBV
- In genotype 1b, treatment for 12 or 24 weeks achieved combined SVR12 rates of 84.9% ( DCV + SMV ) and 74.5% ( DCV + SMV + RBV ) in treatment naïve patients and 69.6% ( DCV + SMV ) and 95.0% (DCV + SMV + RBV ) in prior null responders
- SVR12 rates were higher in patients without NS5A polymorphisms at baseline
- In genotype 1a, DCV + SMV + RBV for 24 weeks achieved SVR12
of 66.7% in treatment-naïve patients. Null responders required intensification with PEG-IFN
- The DCV dose of 30 mg in combination with SMV provided lower than expected DCV exposure, however no relation with SVR12
- DCV + SMV was well tolerated with or without RBV
- The standard 60mg dose of DCV will be utilized in all future studies involving DCV and SMV
- DCV + SMV + RBV is not recommended for the treatment of genotype 1a
- Other available options for HCV genotype 1b infection have surpassed this regimen in terms of efficacy