OSIRIS Study: SMV + PEG-IFN + RBV in genotype 4
Asselah T. PLoS One 2017 Jan 5;12(1):e0168713
Anti-HCV
Simeprevir
Ribavirin
PEG-IFNα 2a
Simeprevir
Ribavirin
PEG-IFNα 2a
Genotype
4
4
Treatment history
Naive
Naive
Cirrhosis
No
No
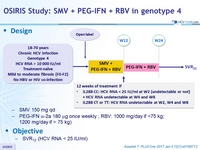
Design
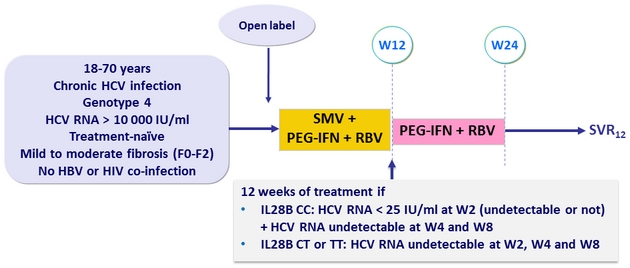
- SMV 150 mg qd
- PEG-IFNα-2a 180 mg once weekly ; RBV: 1000 mg/ day if <75 kg; 1200 mg/ day if > 75 kg)
Objective
- SVR12 (HCV RNA < 15 IU/ml)
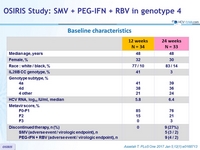
Baseline characteristics
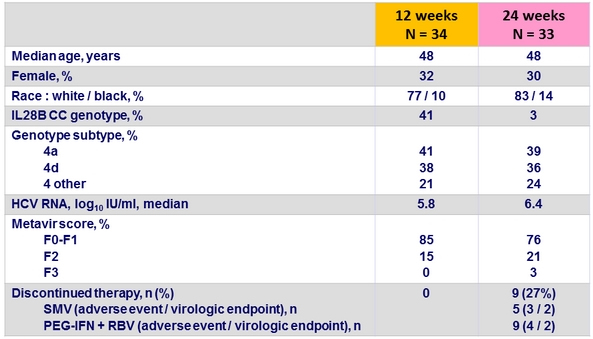
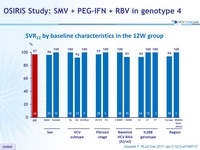
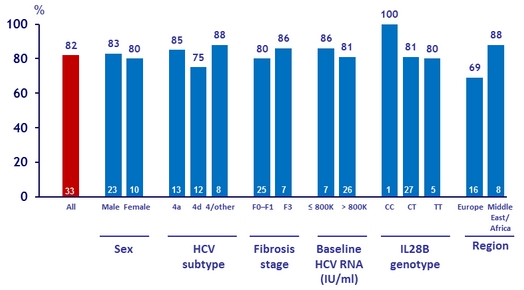
SRV12 by baseline characteristics in the 12W group
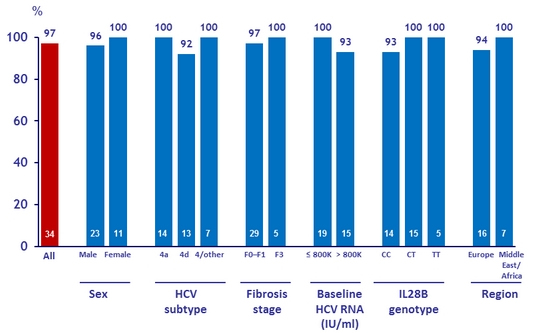
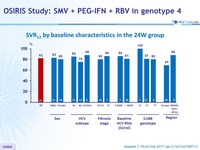
SRV12 by baseline characteristics in the 24W group
Adverse events
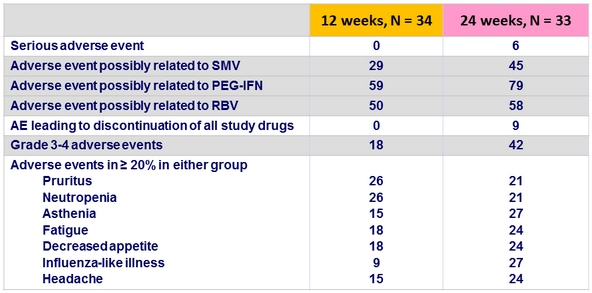
- 2 serious adverse events, not related to SMV: acute sinusitis (N = 1) and phlebitis (N = 1)
- 3 grade 3/4 adverse events were considered related to SMV (all grade 3, all in W24 group): asthenia (N = 2) and increased serum bilirubin (N = 1)
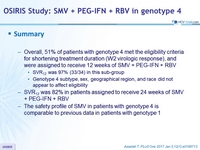
Summary
- Overall, 51% of patients with genotype 4 met the eligibility criteria for shortening treatment duration (W2 virologic response), and were assigned to receive 12 weeks of SMV + PEG-IFN + RBV
- SVR12 was 97% (33 /34) in this sub-group
- Genotype 4 subtype, sex, geographical region, and race did not appear to affect eligibility
- SVR12 was 82 % in patients assigned to receive 24 weeks of SMV + PEG-IFN + RBV
- The safety profile of SMV in patients with genotype 4 is comparable to previous data in patients with genotype 1