SURVEYOR-I Study - Part 1: glecaprevir + pibrentasvir in genotype 1 – Phase II
Kwo PY. J Hepatol 2017; 67 :263-71
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
1
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
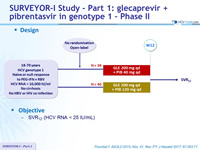
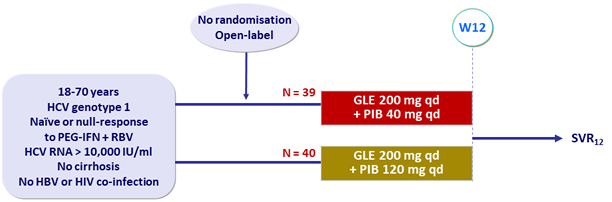
Design
Objective
- SVR12, (HCV RNA < LLOQ)
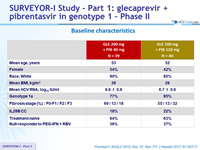
Baseline characteristics
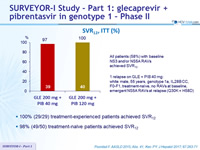
SRV12, ITT (%)
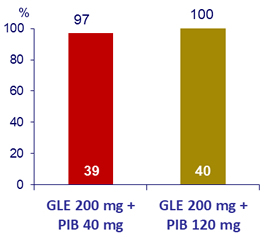 All patients (58%) with baseline NS3 and/or NS5A RAVs achieved SVR12
All patients (58%) with baseline NS3 and/or NS5A RAVs achieved SVR12
1 relapse on GLE + PIB 40 mg: white male, 55 years, genotype 1a, IL28B CC, F0-F1, treatment-naïve, no RAVs at baseline, emergent NS5A RAVs at relapse (Q30K + H58D)
- 100% (29/29) treatment-experienced patients achieved SVR12
- 98% (49/50) treatment-naïve patients achieved SVR12
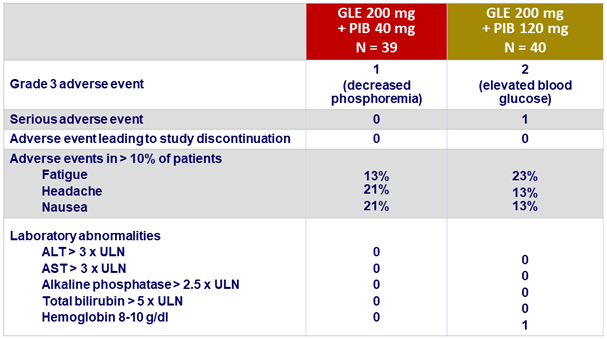
Adverse events and laboratory abnormalities
Summary
- High SVR rates were achieved in HCV genotype 1-infected patients without cirrhosis after 12 weeks of GLE + PIB
- All but one patient achieved SVR12
- 1 relapse in a patient treated with the lowest PIB 40 mg dose
- Adverse events were mostly mild in severity
- Selected doses for future studies: GLE 300 mg qd + PIB 120 mg qd