GARNET Study: OBV/PTV/r + DSV 8 weeks in genotype 1b
Welzel TM. Lancet Gastroenterol Hepatol. 2017; 2:494-500
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Genotype
1b
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
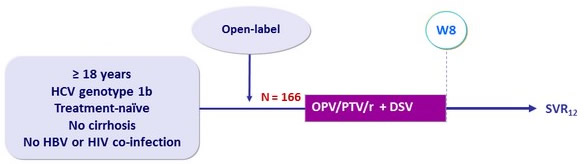
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg QD = 2 tablets
- Dasabuvir (DSV) : 250 mg bid
Objective
- SVR12 (HCV RNA < 15 IU/ml )
- Virologic failures and relapses
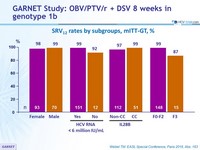
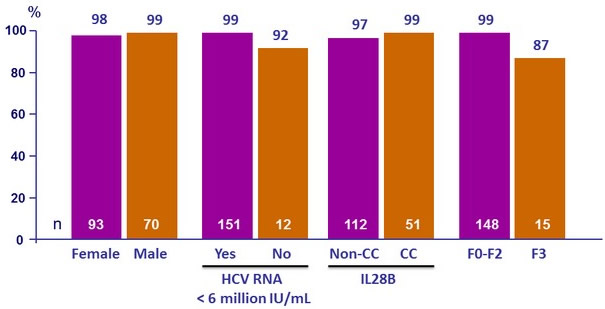
- SVR12 in patients with baseline HCV RNA < 6 000 000 IU/mL
Baseline characteristics and outcome
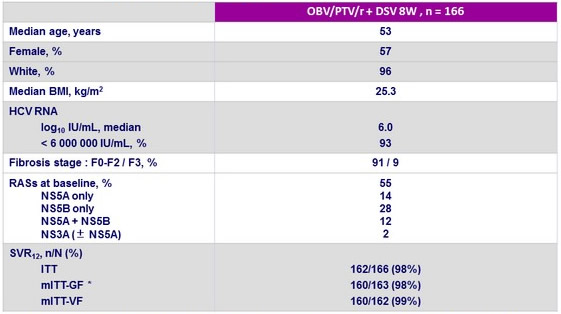
* Exclusion of 3 patients with non-1b genotype : 1 genotype 1a, 1 genotype 1d, 1 genotype 6
** Exclusion of non- virologic failures (1 early discontinuation for non-compliance)
SRV12 rates by subgroups, mITT-GT, %
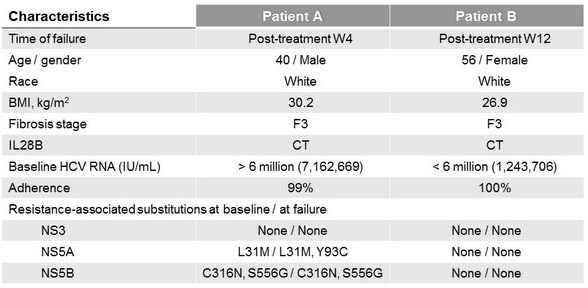
Virologic failures, n = 2 (exclusion of a 3rd failure in a patient with genotype 6)
Treatment-emergent adverse events
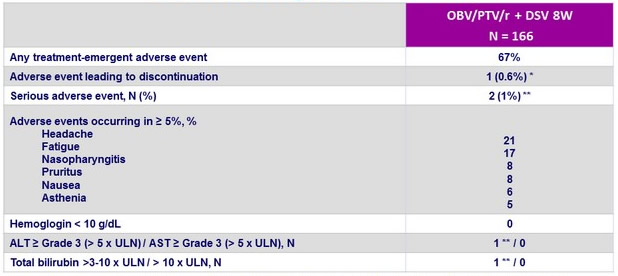
* One 24-year-old female patient with F0–F1 fibrosis discontinued study drug on D45 due to grade 3 hyperbilirubinemia (direct and indirect) that was considered possibly related to study drugs. A grade 3 ALT elevation occurred following hyperbilirubinemia ; both returned to normal. The patient achieved SVR12
** syncope on D17, gastroenteritis on post-treatment D8 ; both were deemed unrelated to study drugs
Summary
- The 3D regimen administered for 8 weeks achieved a 98% SVR12 in treatment-naïve genotype 1b patients without cirrhosis
- Both patients who experienced virologic failure had F3 fibrosis at baseline
- Fibrosis (F3 vs F0–F2) was the only significant predictor of SVR12
- Baseline HCV RNA, sex, BMI, age, and former IV drug use were not predictive of treatment failure
- Presence of resistance-associated polymorphisms at baseline did not impact SVR
- The 8-week, RBV-free 3D regimen was well tolerated
- Most adverse events were mild or moderate in severity
- Serious adverse events and clinically significant laboratory abnormalities were rare (<1% )
- The 98% SVR12 rate demonstrates that treatment- naïve genotype 1b patients without cirrhosis can be eff ecti vely treated with 3D for 8 weeks