CORAL-I study cohorts 3 to 6
Agarwal K, EASL 2017, Abs. FRI-267
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Genotype
1
4
1
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Special population
Liver transplantation
Renal transplantation
Liver transplantation
Renal transplantation
Design
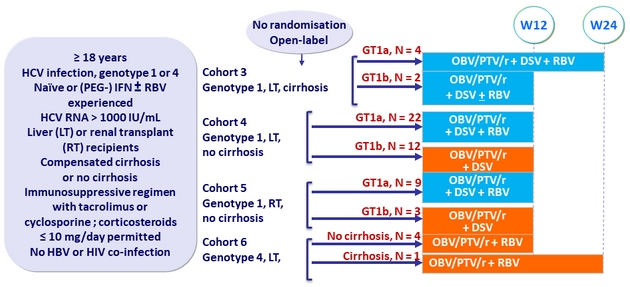
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg QD = 2 tablets
- Dasabuvir (DSV): 250 mg BID ; RBV: 1000 or 12000 mg/day divided twice daily
Objective
- SVR12 (HCV RNA < 25 IU/mL), with 95% CI, by ITT, descriptive analysis
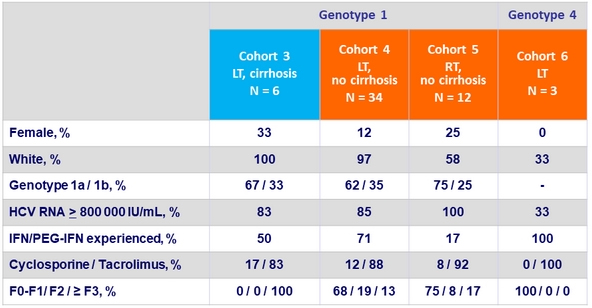
Baseline characteristics
SVR12 by subgroup, ITT population
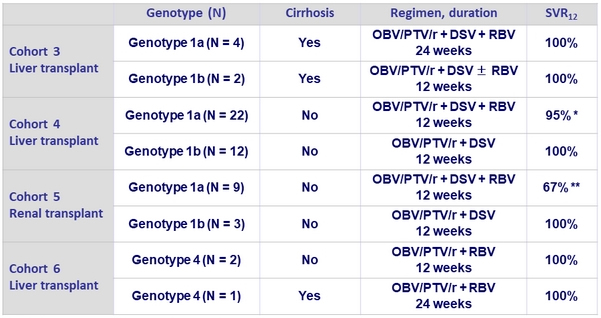
* 1 virologic failure ; ** 3 premature discontinuations
Adverse events, %
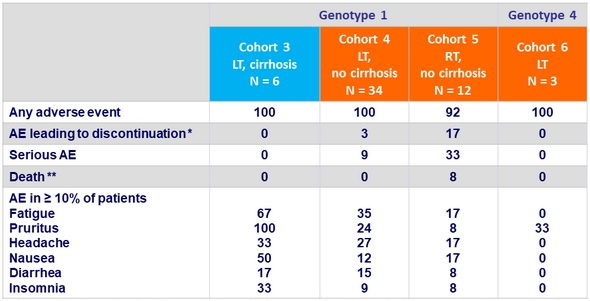
* Nausea and vomiting leading to withdrawal of consent (N = 1), atypical pneumonia with acute renal injury followed by acute respiratory failure (N = 1), and tacrolimus overdose (N = 1)
** 1 patient (tacrolimus overdose)
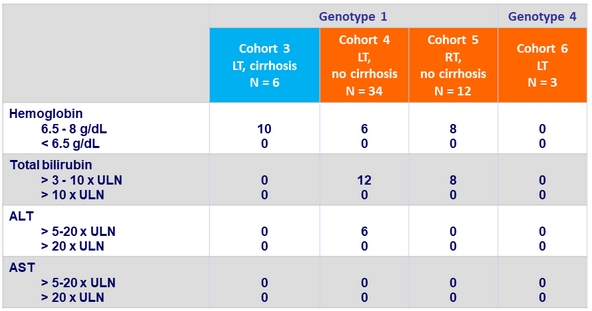
Laboratory abnormalities, %
Summary
- In liver transplant recipients with or without cirrhosis and infected with HCV genotype 1 or 4, treatment with OBV/PTV/r ± DSV ± RBV was associated
- with high SVR12 rates
- and good tolerability
- In renal transplant recipients, SVR12 was achieved in 9/12 patients, with 3 premature discontinuations due to adverse events, including 1 fatality
- Need for tacrolimus dose reduction and therapeutic drug monitoring of tacrolimus levels to manage the interaction with ritonavir
- Patients with genotype 1b achieved SVR12 of 100% with RBV-free regimens






