LDV/SOF Failure Study: LDV/SOF for retreatment of genotype 1 with prior failure to LDV/SOF
Retreatment of Patients who Failed 8 or 12 Weeks of Ledipasvir/Sofosbuvir-based Regimens with Ledipasvir/Sofosbuvir for 24 weeks
Lawitz E. EASL 2015, Abs. O005
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1a
1
1a
Treatment history
NS5A experienced
NS5A experienced
Cirrhosis
Yes
No
Yes
No
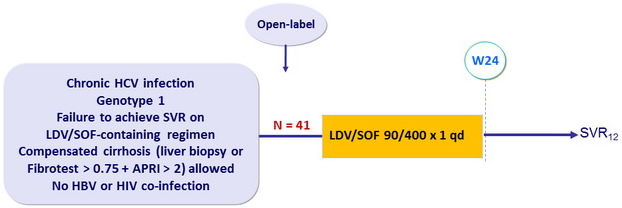
Design
Objective
- Primary endpoint : SVR12 (HCV RNA < 15 IU/ml) by intention to treat, with 2-sided 95% CI, no statistical hypothesis
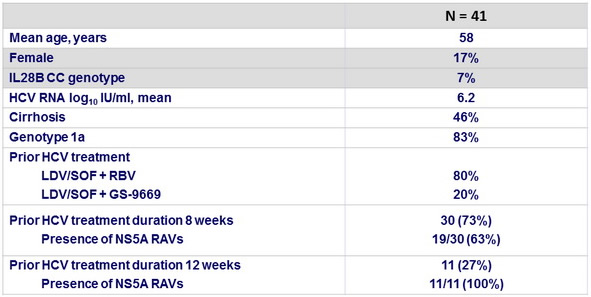
Baseline characteristics
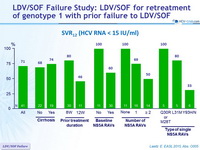
SVR12 (HCV RNA < 15 IU/ mL)
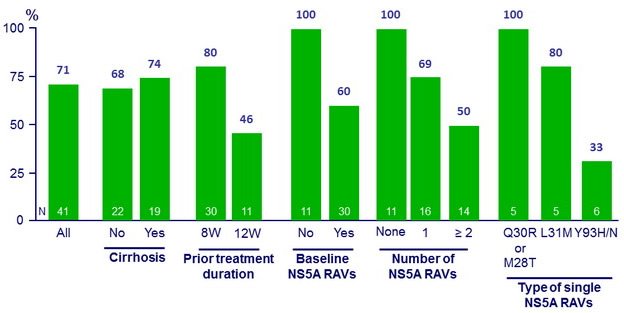
NS5B Resistance analysis
- Baseline : No NS5B resistance associated (S282T) or treatment-emergent (L159F, V321A) variants were detected
- At virologic failure : NS5B variants detected in 4 of 12 (33%) patients
- S282T (N = 2)
- L159F (N = 1)
- Double-mutant S282T + L159F (N = 1)
Adverse events
- Serious adverse events : 3
- Discontinuation due to adverse event : 0
- Grade 3 adverse events : 3 (none related to study drug)
- Adverse events in = 10% of patients : headache, fatigue
- Grade 3 laboratory abnormality : 2
Summary
- 71% of patients who failed prior LDV/SOF-containing regimens achieved SVR12 when retreated with LDV/SOF for 24 weeks
- The presence of baseline NS5A RAV(s), which was more likely to develop with longer prior LDV/SOF treatment, was associated with virologic failure
- Emergence of S282T was observed in 3 of 12 virologic failures