OPTIMIST-1 Study: SMV + SOF for genotype 1 and no cirrhosis
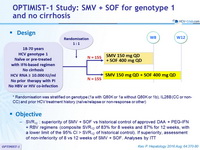
A Phase 3, Randomised, Open-Label Study to Evaluate the Efficacy and Safety of 12 and 8 Weeks of Simeprevir (SMV) plus Sofosbuvir (SOF) in Treatment-Naïve and -Experienced Patients with Chronic HCV Genotype 1 Infection without Cirrhosis: OPTIMIST-1
Kwo P. Hepatology. 2016 Aug; 64:370-80
Anti-HCV
Simeprevir
Sofosbuvir
Simeprevir
Sofosbuvir
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
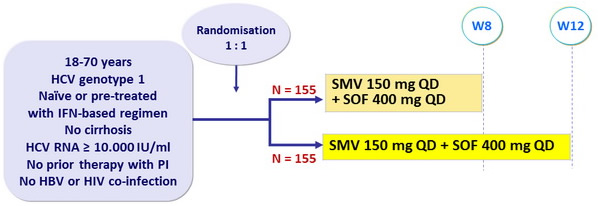
* Randomisation was stratified on genotype (1a with Q80K or 1a without Q80K or 1b), IL28B (CC or non-CC) and prior HCV treatment history (naïve/relapse or non-response or other)
Objective
- SVR12 : superiority of SMV + SOF vs historical control of approved DAA + PEG-IFN + RBV regimens (composite SVR12 of 83% for 8 weeks and 87% for 12 weeks, with a lower limit of the 95% CI > SVR12 of historical control). If superiority, assessment of non-inferiority of 8 vs 12 weeks of SMV + SOF. Analyses by ITT
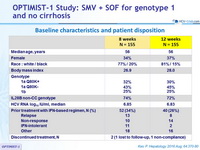
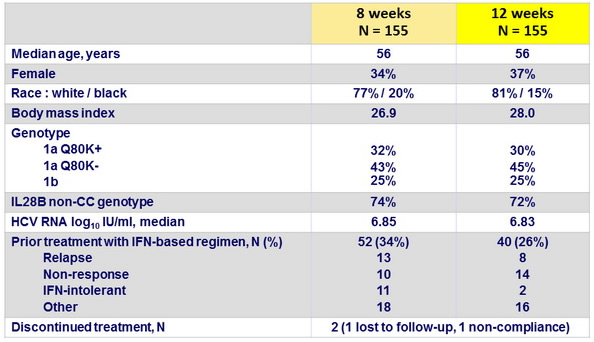
Baseline characteristics and patient disposition
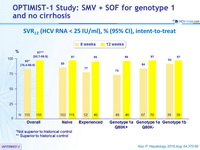
SVR12 (HCV RNA < 25 IU/ml), % (95% CI), intent -to- treat
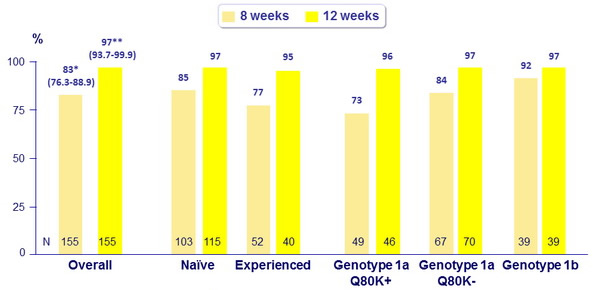
*Not superior to historical control
** Superior to historical control
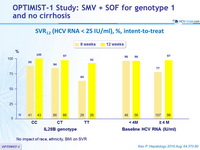
SVR12 (HCV RNA < 25 IU/ml), %, intent -to- treat
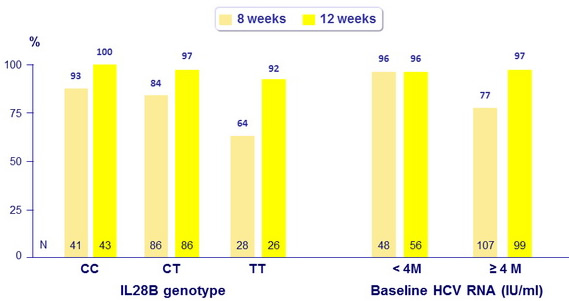
No impact of race, ethnicity, BMI on SVR
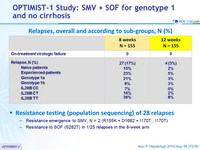
Relapses, overall and according to sub-groups, N (%)
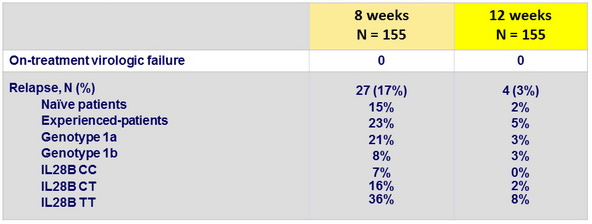
Resistance testing (population sequencing) of 28 relapses
- Resistance emergence to SMV, N = 2 (R155K + D1682 + I170T ; I170T)
- Resistance to SOF (S282T) in 1/25 relapses in the 8-week arm
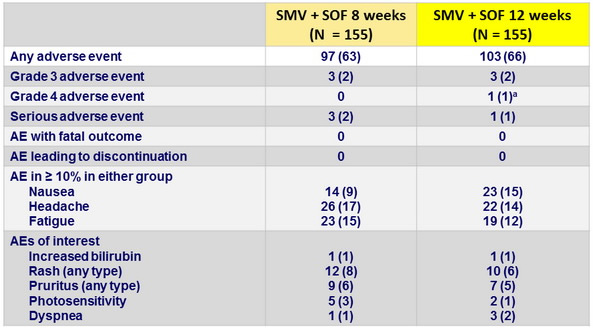
Adverse events, N (%)
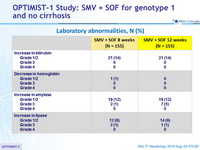
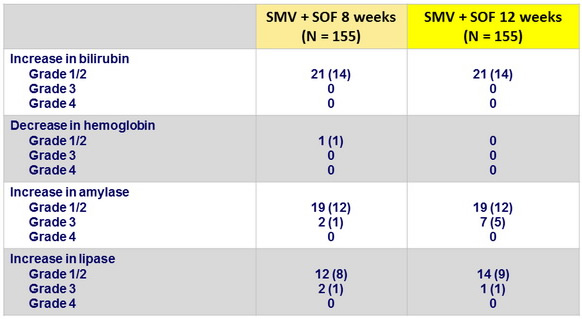
Laboratory abnormalities, N (%)
Summary
- In HCV genotype 1- infected treatment-naïve and treatment-experienced patients without cirrhosis, 12 weeks of SMV + SOF led to SVR12 rates of 97% overall, and demonstrated superiority over the historical control
- 8 weeks of SMV + SOF led to SVR12 rates of 83% overall, and did not achieve superiority compared with the historical control
- In the 12-week arm, SVR12 = 92% were observed in all subgroups, including those with baseline characteristics historically associated with a poor response to HCV treatment (non-CC IL28B genotype, high HCV RNA at baseline, genotype 1a [ with or without Q80K])
- In the 8-week arm, high SVR12 rates were observed in patients with baseline HCV RNA < 4 million IU /ml (96%), genotype 1b ( 92%) and IL28B CC genotype (93%)
- Patients in the 8-week arm with baseline HCV RNA < 4 million IU /ml had low relapse rates (4%)
- Treatment with SMV+SOF for 12 or 8 weeks was safe and well tolerated, with no discontinuations due to adverse events
- Patient-reported symptoms and quality of life significantly improved from baseline to the SVR12 time point in both treatment arms